阿魏酸通过不可逆地结合过氧化物还氧蛋白1抑制其二聚体和分泌,改善tlr4介导的巨噬细胞活化。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:在先天免疫系统中,受损细胞释放的损伤相关分子模式(DAMPs),如过氧化物还氧蛋白1 (PRDX1),与toll样受体4 (TLR4)相互作用,加剧炎症和组织损伤。阿魏酸(FA)是一种膳食和草药酚类化合物,具有显著的抗炎特性。然而,FA的确切抗炎机制尚不完全清楚。目的:本研究的目的是阐明FA抗炎作用的机制。方法:气管内灌注脂多糖(LPS),建立脂多糖性肺炎小鼠模型。腹腔注射不同剂量FA,观察其抗炎作用。炔基修饰的FA探针结合多种化学生物学策略来探索FA靶点的定位、捕获和鉴定。通过严格的生化分析和全面的转录组分析,进一步验证了FA的药效团及其与靶标或途径相互作用的机制。在小鼠腹腔注射LPS的实验中证实了FA抗全身性炎症的机制。结果:FA可选择性靶向巨噬细胞,减轻lps诱导的小鼠肺部炎症。随后,PRDX1被鉴定为不可逆的fa结合靶标。机制研究表明,FA中的α,β-不饱和酮是与PRDX1的Cys173残基共价结合的关键药效团。这种共价结合事件有效抑制了PRDX1二聚化,导致PRDX1分泌减少。共定位实验表明,在lps处理的RAW264.7细胞中,FA降低了tlr4结合的PRDX1。转录组学分析表明,TLR4信号通路下游的NF-κB和TNF信号通路参与了fa介导的抗炎作用。FA处理引起的TLR4活化降低了lps处理的RAW264.7细胞中下游炎症细胞因子的水平。最后,在lps诱导的气管内炎症小鼠的肺切片中,证实了FA减少了TLR4和PRDX1的共定位。在腹腔注射LPS的小鼠中,FA也降低了PRDX1二聚体并减轻了炎症。结论:本研究揭示了FA与PRDX1的Cys173残基共价结合,通过调控PRDX1/TLR4信号通路抑制其二聚体和分泌,从而减轻炎症的新机制。我们的研究结果重新定义了植物苯丙烯酸作为DAMPs的共价调节剂,与传统抗氧化剂相比,具有改善炎症的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
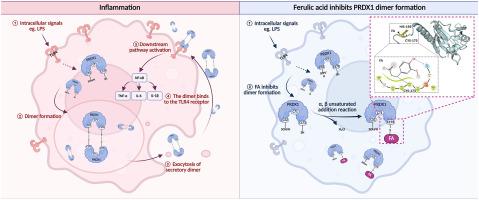
Ferulic acid ameliorates TLR4-mediated macrophage activation by irreversibly binding to peroxiredoxin 1 to inhibit its dimerization and secretion
Background
In the innate immune system, damage-associated molecular patterns (DAMPs) released by damaged cells, such as peroxiredoxin 1 (PRDX1), interact with Toll-like receptor 4 (TLR4), exacerbating inflammation and tissue injury. Ferulic acid (FA), a dietary and herbal phenolic compound, exhibits notable anti-inflammatory properties. However, the precise anti-inflammatory mechanisms of FA are not fully understood.
Purpose
The objective of this study was to elucidate mechanisms underlying the anti-inflammatory effects of FA.
Methods
Lipopolysaccharide (LPS) was intratracheally instilled to establish a mouse model of LPS-induced pneumonia. Different doses of FA were injected intraperitoneally, and their anti-inflammatory effects were evaluated. An alkynyl-modified FA probe was used in conjunction with various chemobiological strategies to explore the localization, capture, and identification of FA targets. The pharmacophore of FA and its mechanisms of interaction with its target or pathway were further validated using rigorous biochemical assays and comprehensive transcriptomic profiling. The proposed mechanism of FA against systemic inflammation was validated in mice administered LPS intraperitoneally.
Results
FA alleviated LPS-induced pulmonary inflammation in mice by selectively targeting macrophages. Subsequently, PRDX1 was identified as an irreversible FA-binding target. Mechanistic investigations revealed that α,β-unsaturated ketone in FA serves as a critical pharmacophore that covalently binds to the Cys173 residue of PRDX1. This covalent binding event effectively suppressed PRDX1 dimerization, resulting in reduced PRDX1 secretion. The co-localization assay demonstrated that FA reduced TLR4-binding PRDX1 in LPS-treated RAW264.7 cells. Transcriptomic analysis indicated that NF-κB and TNF signaling pathways, downstream of TLR4 signaling, were involved in the FA-mediated anti-inflammatory effects. Reduction of TLR4 activation caused by FA treatment decreased levels of downstream inflammatory cytokines in LPS-treated RAW264.7 cells. Finally, reduced co-localization of TLR4 and PRDX1 by FA was confirmed in the lung slices of mice with LPS-induced intratracheal inflammation. FA also reduced PRDX1 dimerization and mitigated inflammation in mice administered LPS intraperitoneally.
Conclusion
Our study elucidated a novel mechanism in which FA covalently binds to the Cys173 residue of PRDX1, suppressing its dimerization and secretion and thereby alleviating inflammation by modulating the PRDX1/TLR4 signaling pathway. Our findings redefine plant phenylacrylic acids as covalent modulators of DAMPs, with therapeutic potential for ameliorating inflammation beyond conventional antioxidants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


