毛缕木中具有抗炎活性的非halimane和spirolabdane二萜。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
11个先前未被描述的二萜,cilileucapenoids A-K(1-10和12),包括2个halimane二萜(1和12)和9个spirorolabdane二萜(2-10),以及一个已知的类似物(11),从Leucas ciliata整个植物中分离出来。通过HR-ESI-MS, 1D/2D NMR,电子圆二色性(ECD)计算,Mosher酯化反应和单晶x射线衍射分析确定了它们的结构和绝对构型。值得注意的是,1是首次报道的芳香卤烷二萜,而2是罕见的双螺-四氢呋喃-labdane骨架。化合物3-10均匀地加入螺-四氢呋喃部分。在生物活性评估中,化合物1、3、4和7通过抑制lps刺激的RAW264.7巨噬细胞一氧化氮(NO)的产生而表现出显著的抗炎作用,IC50值在9.13 ~ 25.94 μM之间。构效关系(SAR)分析进一步证实,相对于e构型,这些二萜类化合物的z构型具有明显增强的抗炎作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
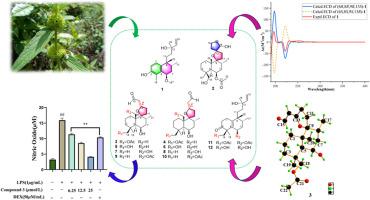
Nor-halimane and spiro-labdane diterpenoids with anti-inflammatory activity from Leucas ciliata Benth
Eleven previously undescribed diterpenoids, cilileucapenoids A–K (1–10 and 12), comprising two halimane diterpenoids (1 and 12) and nine spiro-labdane diterpenoids (2–10), along with one known analogue (11), were isolated from the whole plant of Leucas ciliata. The structures and absolute configurations were determined through the analysis of their HR-ESI-MS, 1D/2D NMR, electronic circular dichroism (ECD) calculations, Mosher's esterification, and single-crystal X-ray diffraction. Notably, 1 represents the first reported aromatic halimane diterpenoid, while 2 features as a rare dispiro-tetrahydrofuran-labdane skeleton. Compounds 3–10 uniformly incorporate a spiro-tetrahydrofuran moiety. In bioactivity assessments, compounds 1, 3, 4, and 7 demonstrated significant anti-inflammatory effects by inhibiting nitric oxide (NO) production in LPS-stimulated RAW264.7 macrophages, with IC50 values ranging from 9.13 to 25.94 μM. Structure-activity relationship (SAR) analysis further established that the Z-configuration of these diterpenoids confers markedly enhanced anti-inflammatory efficacy relative to the E-configuration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


