黑檀烯A-E:黑檀的外消旋二苯乙烯二聚体对糖尿病肾病具有立体特异性抑制作用。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从桑(Morus nigra L.)茎中分离到5个外消旋二苯乙烯二聚体nigraene a - e(1-5),通过HR-ESI-MS、IR和NMR数据对其化学结构进行了鉴定。手性相高效液相色谱分离得到5对对映体,其绝对构型通过量子化学ECD计算得到。所有分离物对高糖(HG)诱导的大鼠肾小球系膜细胞(HBZY-1)增殖的抑制作用进行了评价。在5个外消旋苯乙烯中,2个同源二聚体1和3对hg刺激的HBZY-1增殖表现出明显的抑制作用。(+)-3的IC50 = 2.96 μM,明显优于(-)- 1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
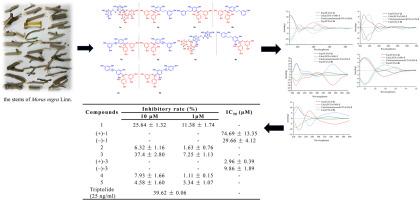
Nigraenes A-E: Racemic stilbene dimers from Morus nigra exhibiting stereospecific suppression of diabetic kidney disease
Five racemic stilbene dimers, nigraenes A-E (1–5), were isolated from the stems of Morus nigra L. Their chemical structures were assigned through a comprehensive analysis of HR-ESI-MS, IR, and NMR data. The chiral-phase HPLC resolution was successfully carried out to yield five pairs of enantiomers, whose absolute configurations were unambiguously determined by quantum chemical ECD calculations. All isolates were evaluated for their inhibitory effects against high glucose (HG)-induced proliferation of rat glomerular mesangial cells (HBZY-1). Among the five racemic stilbenes, two homologous dimers 1 and 3 exhibited significant inhibition against HG-stimulated HBZY-1 proliferation. Notably, (+)-3 showed superior activity (IC50 = 2.96 μM) compared with its (−)-counterpart.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


