上颌触须感受器介导舌蚜避尸。
IF 2.3
2区 农林科学
Q1 ENTOMOLOGY
引用次数: 0
摘要
瓢虫是一种重要的掠食性天敌,具有广泛的食性和较少的食物来源。然而,它对蚜虫尸体表现出回避行为,可能将野外病原体感染的风险降至最低。这种行为的分子基础尚不清楚,并推测味觉受体(GRs)参与其中。在这里,我们研究了豌豆蚜对豌豆蚜虫(Acyrthosiphon pisum)尸体的回避,并研究了候选GR基因在通过上颚触须接触化学接受中的作用。利用扫描电镜和免疫荧光技术对上颌触诊的微观结构进行了表征,并绘制了神经元分布图。行为分析结合RNAi评估了与尸体识别相关的三个候选GR基因。我们的研究结果表明,沙蚤的摄食行为在很大程度上是由上颌触须接触的味觉输入决定的。触须表面密集地覆盖着感受器,内部组织显示出多巴胺能和血清素能神经元的互补分布。RNAi分析表明,三种GR基因(GR2-like, GR28b-like和gr64 -like)介导尸体回避。单独沉默GR2-like可显著降低回避反应,而同时敲低GR28b-like和gr64 -like则进一步削弱了回避反应,提示红毛鼠中味觉受体之间存在功能相互作用。这些发现揭示了食肉昆虫躲避尸体的味觉机制,并为生物防治和人工饮食开发提供了相关见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
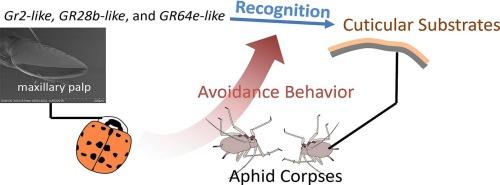
Maxillary palp gustatory receptors mediate aphid corpses avoidance in Harmonia axyridis
The ladybird Harmonia axyridis is an important predatory natural enemy with a broad dietary spectrum and few avoided food sources. Nevertheless, it shows avoidance behavior toward aphid corpses, likely to minimize the risk of pathogen infection in the wild. The molecular basis of this behavior remains unclear, and gustatory receptors (GRs) are hypothesized to be involved. Here, we examined the avoidance of H. axyridis toward pea aphid (Acyrthosiphon pisum) corpses and investigated the role of candidate GR genes in contact chemoreception via the maxillary palps. Using SEM and immunofluorescence, we characterized the microstructure of the maxillary palps and mapped neuronal distributions. Behavioral assays combined with RNAi were performed to evaluate three candidate GR genes associated with corpse recognition. Our results show that feeding behavior on A. pisum is largely determined by gustatory input through maxillary palp contact. The palp surface is densely covered with sensilla, and internally the tissue displays a complementary distribution of dopaminergic and serotonergic neurons. RNAi assays demonstrated that three GR genes (GR2-like, GR28b-like, and GR64e-like) mediate corpse avoidance. Silencing GR2-like alone significantly reduced avoidance, while simultaneous knockdown of GR28b-like and GR64e-like further weakened the response, suggesting functional interactions among gustatory receptors in H. axyridis. These findings reveal a gustatory mechanism underlying corpse avoidance in predatory insects and provide insights relevant to biological control and artificial diet development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of insect physiology
生物-昆虫学
CiteScore
4.50
自引率
4.50%
发文量
77
审稿时长
57 days
期刊介绍:
All aspects of insect physiology are published in this journal which will also accept papers on the physiology of other arthropods, if the referees consider the work to be of general interest. The coverage includes endocrinology (in relation to moulting, reproduction and metabolism), pheromones, neurobiology (cellular, integrative and developmental), physiological pharmacology, nutrition (food selection, digestion and absorption), homeostasis, excretion, reproduction and behaviour. Papers covering functional genomics and molecular approaches to physiological problems will also be included. Communications on structure and applied entomology can be published if the subject matter has an explicit bearing on the physiology of arthropods. Review articles and novel method papers are also welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


