负载他克莫司的纳米结构脂质载体的成膜系统用于局部治疗特应性皮炎。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-16
DOI:10.1016/j.ejpb.2025.114871
引用次数: 0
摘要
他克莫司(TAC)是一种局部用于t细胞介导的皮肤疾病的钙调神经磷酸酶抑制剂,由于溶解度差和皮肤渗透有限,面临着挑战。为了解决这些限制,研究人员开发了一种含纳米结构脂质载体(NLCs)的成膜系统(FFS),以增强局部给药。采用高温高压均质法制备TAC-NLC,并采用Box-Behnken设计优化。优化后的TAC-NLC粒径为102.7 nm, PDI为0.126,包封率为80.5 %,载药量为2.5 %。TAC-NLC@FFS表现出球形形态和改善皮肤粘附性。体外研究显示持续的药物释放和降低细胞毒性。在2,4-二硝基氯苯(DNCB)诱导的特应性皮炎模型中,与阴性对照组相比,TAC-NLC@FFS显著降低了皮炎评分(第7周评分:3.5 ± 0.6 vs. 8.00 ± 1.2),其疗效与商业软膏相当。脾脏重量(全身性炎症指标)在所有治疗组均显著降低,支持抗炎活性。此外,tac治疗组血清IgE和IL-4水平降低,其中IL-4降低具有统计学意义。这些发现表明TAC-NLC@FFS结合了改善皮肤传递和配方稳定性与治疗效果,为局部治疗特应性皮炎提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
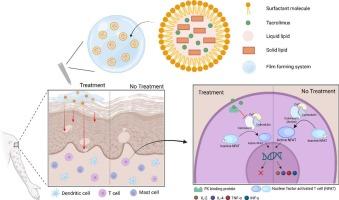
Film-forming system of optimized tacrolimus-loaded nanostructured lipid carriers for effective topical treatment of atopic dermatitis
Tacrolimus (TAC), a calcineurin inhibitor used topically for T-cell-mediated skin diseases, faces challenges due to poor solubility and limited skin penetration. To address these limitations, a film-forming system (FFS) incorporating nanostructured lipid carriers (NLCs) was developed for enhanced topical delivery. TAC-loaded NLCs (TAC-NLC) were prepared via high-temperature and high-pressure homogenization and optimized using Box-Behnken design. The optimized TAC-NLC showed 102.7 nm particle size, 0.126 PDI, 80.5 % encapsulation efficiency, and 2.5 % drug loading. TAC-NLC@FFS demonstrated spherical morphology and improved skin adhesion. In vitro studies showed sustained drug release and reduced cytotoxicity. In a 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis model, TAC-NLC@FFS significantly reduced dermatitis scores compared to the negative control group (score at week 7: 3.5 ± 0.6 vs. 8.00 ± 1.2), with efficacy comparable to commercial ointments. Spleen weight, an indicator of systemic inflammation, was significantly reduced in all treatment groups, supporting anti-inflammatory activity. Additionally, serum IgE and IL-4 levels, key markers of allergic inflammation, were decreased in TAC-treated groups, with IL-4 reduction showing statistical significance. These findings suggest that TAC-NLC@FFS combines improved skin delivery and formulation stability with therapeutic efficacy, offering a promising strategy for the topical treatment of atopic dermatitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


