成像流式细胞术对疟疾感染红细胞的定量和可视化。
IF 2.5
3区 医学
Q2 PARASITOLOGY
引用次数: 0
摘要
虽然传统上认为疟疾寄生虫会感染无核红细胞,但最近的证据——包括我们之前的工作——表明它们能够侵入有核红细胞前体(红母细胞)。然而,由于其在外周血中的罕见性和现有技术的局限性,体内寄生红细胞(pEBs)的可视化和量化仍然具有挑战性。在本研究中,我们采用成像流式细胞术(ImageStream)对表达绿色荧光蛋白的约氏疟原虫17XNL感染小鼠的pEBs进行鉴定和表征,并与常规流式细胞术(FACS)进行比较。感染后14 d,脾、骨髓和外周血细胞染色抗TER119(红系标记物)和MHC I类抗体,该抗体在有核宿主细胞上表达。为了确认细胞核的存在,我们还使用了Hoechst 33342 DNA染色。ImageStream分析实现了GFP + TER119 + MHC类Ihi Hoechst+ pEBs的同时检测和形态可视化。虽然在外周血中未检测到peb,但在脾脏和骨髓中发现了大量peb。重要的是,ImageStream对pEB频率的量化与FACS的结果非常吻合,验证了基于成像方法的鲁棒性。值得注意的是,ImageStream还发现了涉及peb和MHC I类+细胞的双体和三体,暗示了红母细胞岛或免疫相互作用。偶见泡状结构,提示细胞凋亡样过程。这些发现表明,ImageStream为疟疾中寄生红母细胞的高通量、基于图像的检测提供了一个强大的平台。这种方法可能为研究造血组织内宿主-寄生虫相互作用及其在寄生虫持续存在和免疫逃避中的作用开辟新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
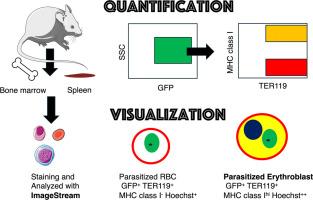
Quantification and visualization of malaria-infected erythroblasts by imaging flow cytometry
Although malaria parasites are traditionally known to infect enucleated red blood cells, recent evidence—including our previous work—demonstrates their ability to invade nucleated erythroid precursors (erythroblasts). However, visualizing and quantifying parasitized erythroblasts (pEBs) in vivo remains challenging due to their rarity in peripheral blood and the limitations of existing techniques. In this study, we employed imaging flow cytometry (ImageStream) to identify and characterize pEBs in mice infected with Plasmodium yoelii 17XNL expressing green fluorescent protein and compared with conventional flow cytometry (FACS). At 14 days post-infection, splenic, bone marrow and peripheral blood cells were stained with antibodies against TER119 (erythroid marker) and MHC class I, which is expressed on nucleated host cells. To confirm the presence of nuclei, we also used Hoechst 33342 DNA staining. ImageStream analysis enabled simultaneous detection of GFP⁺TER119⁺MHC class Ihi Hoechst+ pEBs and morphological visualization. While no pEBs were detected in peripheral blood, a significant population was identified in the spleen and bone marrow. Importantly, quantification of pEB frequencies by ImageStream closely matched those obtained by FACS, validating the robustness of the imaging-based approach. Notably, ImageStream also revealed doublets and triplets involving pEBs and MHC class I⁺ cells, suggestive of erythroblastic islands or immunological interactions. Blebbing structures were occasionally observed, indicating apoptosis-like processes. These findings demonstrate that ImageStream offers a powerful platform for high-throughput, image-based detection of parasitized-erythroblasts in malaria. This approach may open new avenues for studying host–parasite interactions within hematopoietic tissues and their roles in parasite persistence and immune evasion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta tropica
医学-寄生虫学
CiteScore
5.40
自引率
11.10%
发文量
383
审稿时长
37 days
期刊介绍:
Acta Tropica, is an international journal on infectious diseases that covers public health sciences and biomedical research with particular emphasis on topics relevant to human and animal health in the tropics and the subtropics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


