亚纳米Ir/γ-Al2O3上的原子尺度氢动力学:化学吸附、溢出和理论-实验相结合的反向溢出
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
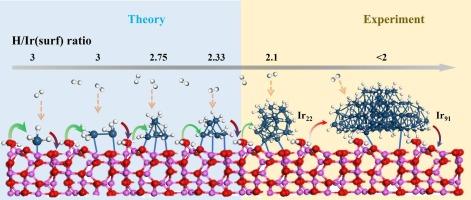
原子尺度上对氢的化学吸附、溢出和金属氧化物支撑的金属(亚)纳米颗粒的反向溢出的深入了解对于推进氢相关技术至关重要。采用计算-实验相结合的方法,我们系统地研究了水合γ-Al2O3(110)负载的Irn亚纳米粒子(n = 1,2,4,6,22)和Ir91纳米粒子的这些过程。采用密度泛函理论(DFT)研究了Ir(亚)纳米颗粒尺寸对氢化学吸附、溢出和反向溢出过程的影响。最大H/Ir(surf)吸附比随着团簇尺寸的增加而减小(饱和比:Ir1为3,Ir22为2.11,Ir91为<;2),并伴随着高H负载下显著的(亚)纳米级金属变形。大多数氢溢出在热力学上是不利的。反向溢出有利于许多支持的Irn集群(n = 1,2,4,6)。热力学分析表明,在中等H2压力(0.01 atm)和925 K的温度下,小Irn簇上的部分氢保留是由于在欠配位的Ir位点上的强结合。H2温度程序还原/解吸(H2- tpr /TPD)实验证实了计算的见解。H2-TPR曲线中~ 518、709和809 K处的峰分别属于表面IrO2、界面Ir-O-Al和ir诱导的缺氧Al2O3的还原。这项工作阐明了支持(亚)纳米金属团簇上氢相互作用的基本原子尺度性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Atomic-scale hydrogen dynamics on (sub)nano-sized Ir/γ-Al2O3: chemisorption, spillover, and reverse spillover via combined theory-experiment
Atomic-scale insights into hydrogen chemisorption, spillover, and reverse spillover on metal-oxide-supported metal (sub)nanoparticles are crucial for advancing hydrogen-related technologies. Using a combined calculations-experiments approach, we systematically studied these processes on hydrated γ-Al2O3(110) supported Irn subnanoparticles (n = 1, 2, 4, 6, 22) and Ir91 nanoparticle. A density-functional theory (DFT) investigation into the influence of Ir (sub)nanoparticles size on the processes of hydrogen chemisorption, spillover, and reverse spillover was initially performed. The maximum H/Ir(surf) adsorption ratio decreases with increasing cluster size (saturation ratios: 3 for Ir1, 2.11 for Ir22, and <2 for Ir91), accompanied by significant (sub)nano-sized metal deformation under high H-loading. Most hydrogen spillover is thermodynamically unfavorable. Reverse spillover is favored for many supported Irn clusters (n = 1, 2, 4, 6). Thermodynamic analysis indicates partial hydrogen retention on small Irn clusters under moderate H2 pressure (0.01 atm) and temperatures at 925 K, due to strong binding at undercoordinated Ir sites. H2 temperature-programmed reduction/desorption (H2-TPR/TPD) experiments confirm computational insights. Peaks at ∼518, 709, and 809 K in H2-TPR curves are assigned to the reduction of surface IrO2, interfacial Ir-O-Al species, and Ir-induced oxygen-deficient Al2O3, respectively. This work elucidates the fundamental atomic-scale nature of hydrogen interactions on supported (sub)nanometer metal clusters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


