合适的碱阳离子改善了Mn(I)催化酯的加氢反应:碱和配体的环大小对催化性能的影响
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
钳形Mn(I)-配合物(Mn-1 - Mn-9)含有二茂铁基pnn -配体家族,其环烷基大小在5到8之间,成功地用作酯加氢的有效催化剂。其中Mn-1与含10 mol%碱的Li+或Na+(尤其是NaBH4和LiH)结合时活性最高,进一步证实了其活化H2的最低吉布斯自由能值。对照实验和DFT计算强调了阳离子和/或阴离子在锰催化氢化氢的异裂解裂解和氢化物转移过程中的作用和规律。特别是以NaBH4和LiH为碱,以Mn-1为催化剂,研究和计算了它们的比较性能,提出了碱阳离子参与加氢途径的两种可能循环。采用合适的碱阳离子对Mn-H催化剂进行强化,将37种不同的芳香族和脂肪族酯加氢为相应的大醇和细醇,其周转量(TON)可达6000。本文章由计算机程序翻译,如有差异,请以英文原文为准。
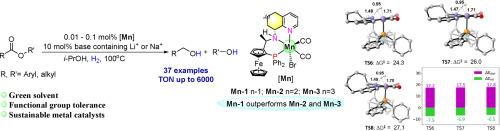
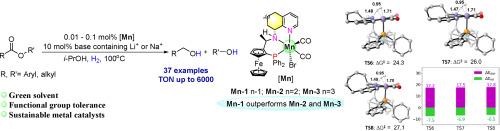
Suitable alkali cations improved Mn(I)-catalyzed hydrogenation of esters: An insight into the effect of bases and ligands’ ring sizes on catalytic performance
Pincer Mn(I)-complexes (Mn-1–Mn-9) bearing a family of ferrocenyl-based PNN-ligands containing the cycloalkyl anywhere ring sizes of from five to eight, were successfully employed as efficient catalysts for ester hydrogenation. Among them, Mn-1, in combination with 10 mol% base-containing Li+ or Na+ (especially NaBH4 and LiH), proved to be the highest activity catalyst, further confirming its lowest Gibbs free energy values for H2 activation. Control experiments and DFT calculations highlight the role and rule of cations and/or anions of bases during heterolytic H2 cleavage and hydride transfer process for Mn-catalyzed hydrogenation. In particular, either NaBH4 or LiH as base, their comparative performance was studied and calculated with Mn-1 as catalyst, two possible cycles of alkali cations participating in the hydrogenation pathway were proposed. Employing suitable alkali cations enhancing the Mn-H catalyst, various aromatic and aliphatic esters (37 examples) were hydrogenated to the corresponding bulk and fine alcohols, offering a turnover number (TON) reaching up to 6000.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


