双功能c2对称手性氯霉素基磷二胺作为对映选择性酰基转移反应的高活性氢键供体有机催化剂
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
制备了一种高效、结构设计良好、可循环利用的c2对称叔胺-磷二胺有机催化剂,用于对映选择性酰基转移反应,可实现立体控制和反应活性调节。在2 摩尔%的有机催化剂存在下,各种中环酐与醇反应,产生相应的手性化合物,收率高(高达97 %),立体控制性好(ee,高达99.8 %)。该方法可以很容易地扩展到(S)-普瑞巴林手性药物前体的合成,收率高达90% %,ee高达99.8 %,克服了使用高催化剂负载的一些传统限制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
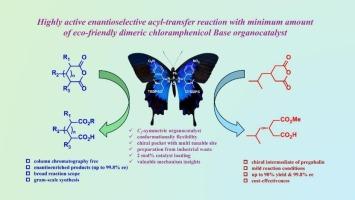
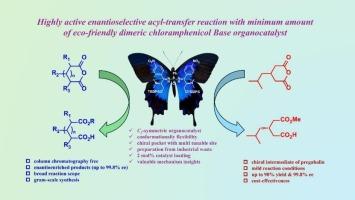
Bifunctional C2-symmetric chiral chloramphenicol base-phosphorodiamides as highly active hydrogen bond donor organocatalysts for enantioselective acyl-transfer reaction
A highly efficient, structurally well-designed and recyclable C2-symmetric tertiary amine-phosphorodiamide organocatalyst for enantioselective acyl-transfer reaction has been elaborated, allowing for stereocontrol and tuning of reactivity. In the presence of only 2 mol% of this organocatalyst, various meso-cyclic anhydrides reacted with alcohol, affording the corresponding chiral hemiesters in high yield (up to 97 %) with excellent stereocontrol (ee, up to 99.8 %). This protocol can be readily extended for the synthesis of chiral drug precursor of (S)-pregabalin with up to 90 % yield and 99.8 % ee, overcoming some of the traditional limitations of using high catalyst loading.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


