双吖啶衍生物作为有效的真核拓扑异构酶II抑制剂
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
真菌感染,特别是由念珠菌引起的真菌感染,由于对常规抗真菌药物的耐药性日益增加,以及通常这些药物的药库有限,构成了越来越大的临床挑战。在这项研究中,我们合成并测试了一系列新的双吖啶衍生物,作为潜在的抗真菌化合物,靶向真菌拓扑异构酶II。这些化合物被评估为它们抑制酵母拓扑异构酶II的能力,它们的抗真菌活性,以及它们对人类酶的选择性。酶促实验证实,其中,化合物IKE16对酵母拓扑异构酶II具有高水平的抑制作用,比其对人具有更高的选择性,并且在体外具有中等的抗真菌活性。基于计算机方法,我们提出了这种行为的机制。特别是,分子对接研究表明,我们的化合物表现出特异性的“非依托泊苷样”类型的酵母拓扑异构酶II抑制。此外,在人酶的情况下,化合物比酵母对应物更少地适应于潜在的结合位点。因此,这可能导致它们对人拓扑异构酶II的活性降低。这些发现突出了双吖啶作为一种潜在的新型抗真菌药物具有新的作用机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
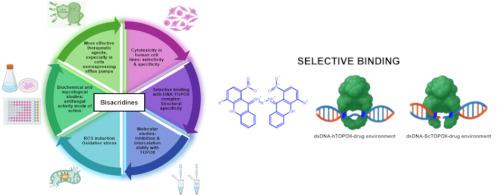
Bisacridine derivatives as effective eukaryotic topoisomerase II inhibitors
Fungal infections, particularly those caused by Candida species, pose a growing clinical challenge due to the increasing resistance to conventional antifungal agents and, in general, the limited arsenal of such drugs. In this study, we synthesized and tested a series of novel derivatives of bisacridines as potential antifungal compounds targeting fungal topoisomerase II. These compounds were evaluated for their ability to inhibit yeast topoisomerase II, their antifungal activity, and their selectivity over the human enzyme. Enzymatic assays confirmed that among them, compound IKE16 exhibited a high level of yeast topoisomerase II inhibition with greater selectivity over its human counterpart, as well as a moderate antifungal activity in vitro. Based on in silico approaches, we propose mechanism for this behavior. In particular, molecular docking studies revealed that our compounds exhibit specific “non etoposide-like” type of yeast topoisomerase II inhibition. Moreover, in case of the human enzyme, the compounds are less accommodated to the potential binding sites than in the yeast counterpart. In consequence it may lead to their lower activity against human topoisomerase II. These findings highlight bisacridines as a potential new class of antifungal agents with a novel mechanism of action.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


