核苷酸药物传递的通用纳米平台:从分子结合到抗病毒治疗
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
核苷类药物是一类重要的治疗病毒感染和恶性肿瘤的药物。它们的药理活性形式,核…本文章由计算机程序翻译,如有差异,请以英文原文为准。
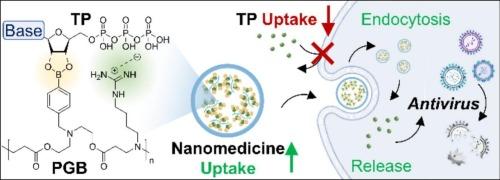
A general nanoplatform for nucleotide drug delivery: From molecular binding to antiviral therapy
Nucleoside-based agents represent an important class of therapeutics for viral infections and malignancies. Their pharmacologically active forms, nucleoside triphosphates (TPs), exhibit high polarity and negative charge severely limiting cellular uptake and intracellular utilization. While prodrug strategies used in clinical practice often exhibit limited activation and efficacy, nanocarrier-based TP delivery offers a promising alternative; however, efficient encapsulation of these highly hydrophilic small molecules remains a great challenge. To address this, we developed a rationally designed polymeric carrier PGB, featuring alternating phenylboronic acid and guanidinium groups that bind to conserved nucleotide motifs through multivalent interactions. This design achieves an association constant up to 5.19 × 108 with ATP at pH 7.4, facilitates efficient cytosolic delivery, and enhances antiviral efficacy across multiple viral infection models. In an H1N1-infected mouse model, the nanoparticle formulation demonstrated a markedly improved pharmacokinetic profile compared to the antiviral drug Molnupiravir, effectively suppressed viral replication, reduced pulmonary inflammatory cell infiltration, and preserved lung structure and function. Our study successfully achieved the efficient loading and intracellular delivery of hydrophilic molecules, providing a versatile and broadly applicable platform for nucleotide drug delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


