艾司西酞普兰的流线型原子经济合成:公斤级工艺优化和工业规模实施
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
一种简单、高效、原子经济、可扩展的商业制造工艺被开发出来,用于通过商业可用的起始材料治疗抑郁症。西酞普兰是一种选择性5 -羟色胺再摄取抑制剂,于1989年推出,是一种外消旋混合物,其全部抑制活性存在于S-(+)-对映体中,也称为艾司西酞普兰。虽然最初的六步法存在总收率低于30%、二醇分解效率低(40%)、溶剂不可回收和反应副产物管理问题,但我们重新设计的工艺实现了三个关键进步:(1)控制杂质分布、提高反应效率(每步收率超过90%)、简化后处理和制备外消旋二醇的溶剂可回收;(2) r -二醇副产物经SN2转化为艾司西酞普兰的立体可逆环化策略;(3)艾司西酞普兰对映体副产物的闭环解析剂回收与评价。安全优化的放大生产使446公斤批次具有卓越的纯度(>99.7%),对映体过量(>99.8% ee)和81.6%的总收率──与传统方法相比,工艺强度提高了3.9倍。这个可持续发展的平台展示了前所未有的原子利用效率(>90%),同时消除了立体化学废物,为艾司西酞普兰的制造建立了新的基准。本文章由计算机程序翻译,如有差异,请以英文原文为准。
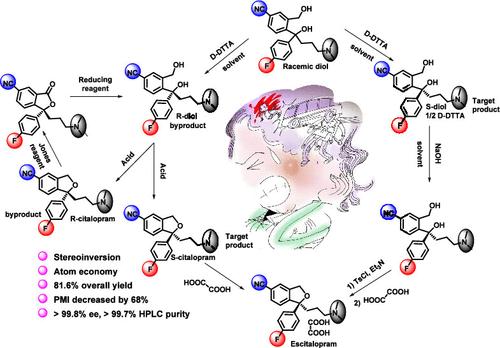
Streamlined Atom-Economical Synthesis of Escitalopram: Kilogram-Scale Process Optimization and Industrial-Scale Implementation
A straightforward, efficient, atom-economical, and scalable commercial manufacturing process was developed for the treatment of depression via commercial available starting materials. Citalopram, a selective serotonin reuptake inhibitor introduced in 1989, is a racemic mixture whose entire inhibitory activity resides in the S-(+)-enantiomer, also known as escitalopram. While the original six-step route suffered from sub-30% overall yield, inefficient diol resolution (<40%), nonrecyclable solvents, and problematic reaction byproduct management, our redesigned process achieves three critical advancements: (1) controlled impurity profiles, enhanced reaction efficiency (over 90% yield per step), streamlined postprocessing and recyclable solvents for preparation of racemic diol; (2) effective stereoinvertive cyclization strategy for converting R-diol byproducts into escitalopram via SN2; (3) closed-loop resolution agent recovery and valorization of escitalopram enantiomeric byproduct. Safety-optimized scale-up enabled production of 446 kg batches with exceptional purity (>99.7%), enantiomeric excess (>99.8% ee), and 81.6% overall yield─representing 3.9-fold process intensification versus legacy methods. This sustainable platform demonstrates unprecedented atom utilization efficiency (>90%) while eliminating stereochemical waste, establishing new benchmarks for escitalopram manufacturing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


