深入了解电解质对电解Fe2+活化过氧单硫酸盐的作用:用于增强净化和可持续电解的硅酸盐
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
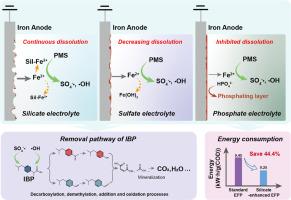
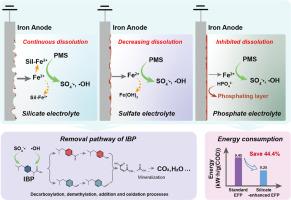
电解Fe2+活化过氧单硫酸盐(EFP)是一种很有前途的去除废水中有机污染物的技术。然而,电解质对EFP性能的影响还有待深入探讨。本研究系统地研究了各种无机阴离子(作为支撑电解质)对EFP体系中铁溶解行为和布洛芬(IBP)降解的影响。实验结果表明,含硅酸盐电解质的EFP体系保持了铁的连续溶解,具有良好的去除IBP的性能。有利于去除IBP的电解质顺序为:硅酸盐;硫酸盐;硝酸盐;氯化物;碳酸氢盐;磷酸盐。具体来说,硅酸盐可以与铁阳极释放的Fe2+离子配合,从而防止电极结垢。同时,EFP中生成的SO4-·和·OH等活性物质对IBP的氧化起主要作用。基于硅酸盐的积极作用,提出了硅酸盐增强EFP的进一步优化方案。EFP的降解与电流密度和PMS浓度显著相关,EFP系统的性能可以通过电流调节。将硅酸盐强化EFP应用于实际制药废水的处理中,不仅表现出去除COD的能力,而且比能耗比显著降低,比能耗比无硅酸盐EFP降低44.4%。总的来说,本研究强调了含硅酸盐电解质在EFP系统和其他使用铁阳极的电化学废水处理系统中的优越性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insight into the role of electrolytes on the electrolytic Fe2+-activated peroxymonosulfate: Silicates for enhanced decontamination and sustainable electrolysis
Electrolytic Fe2+-activated peroxymonosulfate (EFP) is a promising technology for removing organic pollutants from wastewaters. However, the impact of electrolytes on the performance of EFP needs to be explored in depth. This study systematically investigated the impacts of various inorganic anions (as supporting electrolytes) on the iron dissolution behavior and ibuprofen (IBP) degradation within the EFP system. The experimental results revealed that the EFP system with a silicate electrolyte maintained continuous iron dissolution and demonstrated favorable performance in the removal of IBP. The order of electrolytes favoring IBP removal was as follows: silicate > sulfate, nitrate, chloride > bicarbonate > phosphate. Specifically, silicate could coordinate with the Fe2+ ions that were released from the iron anode, thereby preventing the scaling of electrode. Meanwhile, the generated reactive species in the EFP, such as SO4-· and ·OH, were responsible for the oxidation of IBP. Based on the positive effect of silicate, a silicate-enhanced EFP was proposed for further optimization. The degradation of IBP was significantly correlated with the electrical current density and the PMS concentration, and the performance of EFP system can be regulated with electrical current. When the silicate-enhanced EFP was applied to the treatment of actual pharmaceutical wastewater, it not only showed the capability of removing COD, but also notably reduced the specific energy consumption by up to 44.4 %, compared to the silicate-free EFP system. Overall, this study highlights the superiority of silicate-containing electrolytes for the EFP system and in other electrochemical wastewater treating systems using iron anode.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


