血管机械力与血管疾病
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
血管持续受到机械力的作用,主要包括剪切应力、循环拉伸和静水压力。这些力通过复杂的机械传感和信号转导途径调节内皮细胞(ECs)和血管平滑肌细胞(VSMCs)的功能,对维持血管稳态至关重要。然而,在病理条件下,它们可以促进血管疾病的发展,如动脉粥样硬化、高血压和主动脉瘤。本文综述了内皮细胞和VSMCs中血管机械力的传感器和下游信号通路,重点介绍了它们对细胞行为的影响及其在动脉粥样硬化、高血压和主动脉瘤发生和发展中的作用。综述关键科学概念多种分子和结构——包括离子通道、g蛋白偶联受体、细胞连接分子和其他膜结构——作为血管机械力的机械传感器,触发多种下游信号转导途径。切应力、循环拉伸和静水压力的病理改变调节着内皮细胞和VSMCs的功能和行为,包括细胞增殖、迁移、凋亡、氧化应激、内皮通透性等。这些反应诱导血管炎症、功能障碍和重塑,最终导致动脉粥样硬化、高血压和主动脉瘤的发生和发展。本文还强调了静水压力在动脉粥样硬化和高血压中的作用被低估,以及其他研究空白和血管机械力研究的未来方向。了解和治疗调节这些生物力学途径可能最终促进更有效的预防和治疗血管疾病本文章由计算机程序翻译,如有差异,请以英文原文为准。
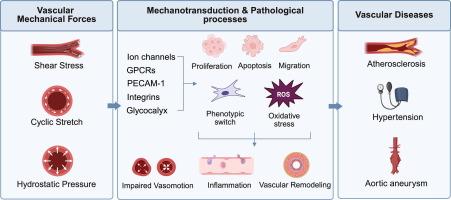
Vascular mechanical forces and vascular diseases
Background
Blood vessels are continuously exposed to mechanical forces, mainly including shear stress, cyclic stretch, and hydrostatic pressure. These forces regulate the functions of endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) through complex mechanosensing and signal transduction pathways, which are essential for maintaining vascular homeostasis. However, under pathological conditions, they can contribute to the development of vascular diseases, such as atherosclerosis, hypertension, and aortic aneurysm.Aim of review
This review aims to synthesize the mechanosensors and downstream signaling pathways of vascular mechanical forces in ECs and VSMCs, emphasizing their effects on cell behaviors and their involvement in the onset and progression of atherosclerosis, hypertension and aortic aneurysms.Key scientific concepts of review
Multiple molecules and structures – including ion channels, G-protein coupled receptors, cellular junction molecules, and other membrane structures – act as mechanosensors of vascular mechanical forces and trigger multiple downstream signal transduction pathways. The pathological alterations in shear stress, cyclic stretch, and hydrostatic pressure regulate the functions and behaviors of ECs and VSMCs, including cellular proliferation, migration, apoptosis, oxidative stress, endothelial permeability, etc. These responses induce vascular inflammation, dysfunction and remodeling, which eventually contributes to the onset and progression of atherosclerosis, hypertension, and aortic aneurysms. This review also highlights the underestimated role of hydrostatic pressure in atherosclerosis and hypertension, as well as other research gaps and future directions for vascular mechanical forces research. Understanding and therapeutically modulating these biomechanical pathways may ultimately facilitate more effective prevention and treatment of vascular diseases求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


