肿瘤相关巨噬细胞氨基酸代谢的动态重塑:促进免疫抑制,重塑肿瘤生态位,解锁代谢检查点
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
肿瘤相关巨噬细胞依赖于其氨基酸代谢来决定其性质和免疫功能,并在肿瘤微环境(TME)中发挥重要作用。虽然在以往的研究中,靶向氨基酸代谢将肿瘤相关巨噬细胞(tumor-associated macrophages, tam)的肿瘤功能转化为抗肿瘤免疫功能已显示出很好的肿瘤治疗应用前景,但目前的临床研究仍然有限。目前缺乏对通过控制tam中氨基酸代谢来决定肿瘤进展的机制和治疗策略的探讨,也缺乏对通过重塑tam中氨基酸代谢来促进肿瘤进展的研究总结。本文旨在系统回顾和总结tam中氨基酸代谢与TME之间的串扰,分析其代谢网络在肿瘤发生发展中的决定作用,并在此基础上总结治疗方法,以确定tam中氨基酸代谢在肿瘤治疗中的发展现状和新兴技术。这篇综述剖析了tam如何通过转运体、酶和传感器利用氨基酸动力学来采用原肿瘤表型,消耗关键代谢物和削弱抗肿瘤t细胞反应。我们绘制了tam重塑免疫的免疫代谢串扰图,强调了营养竞争和代谢副产物是免疫功能障碍的双重驱动因素。针对这些通路(IFN-γ-JAK-STAT1和IL-6/JAK2/STAT3)的新兴治疗策略已被严格评估其重编程tam和逆转免疫抑制的潜力。关键的挑战,如TAM异质性,代谢可塑性和治疗抗性,强调需要单细胞分辨率的TAM代谢状态映射,以确定环境依赖的脆弱性。最后,我们提倡将代谢重组与免疫疗法结合起来的组合方法,提出破坏tam中的氨基酸依赖性可以拆除免疫抑制性TME。本文章由计算机程序翻译,如有差异,请以英文原文为准。
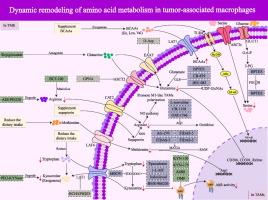
Dynamic remodeling of amino acid metabolism in tumor-associated macrophages: Fueling immunosuppression, reshaping tumor niches, and unlocking metabolic checkpoints
Background
Tumor-associated macrophages depend on their amino acid metabolism to determine their properties and immune function and play important roles in the tumor microenvironment (TME). Although in previous studies, targeting amino acid metabolism to transform the protumor function of tumor-associated macrophages (TAMs) into antitumor immune function has shown promising application as a tumor therapy, current clinical research is still limited. There is a lack of discussion on the mechanism and treatment strategy for determining tumor progression by controlling amino acid metabolism in TAMs, as does a summary of studies on promoting tumor progression by reshaping amino acid metabolism in TAMs.Aim of review
This review aims to systematically review and summarize the crosstalk between amino acid metabolism in TAMs and the TME, analyze the determining role of its metabolic network in tumor occurrence and development, and summarize therapies on this basis to help determine the development status and emerging technologies in the field of amino acid metabolism in TAMs for tumor therapy.Key scientific concepts of review
This review dissects how TAMs exploit amino acid dynamics via transporters, enzymes, and sensors to adopt protumoral phenotypes, depleting critical metabolites and crippling antitumor T-cell responses. We map the immunometabolic crosstalk through which TAMs reshape immunity, highlighting nutrient competition and metabolic byproducts as dual drivers of immune dysfunction. Emerging therapeutic strategies targeting these pathways (IFN-γ-JAK-STAT1 and IL-6/JAK2/STAT3) have been critically evaluated for their potential to reprogram TAMs and reverse immunosuppression. Key challenges, such as TAM heterogeneity, metabolic plasticity, and therapy resistance, are addressed, emphasizing the need for single-cell-resolution mapping of TAM metabolic states to identify context-dependent vulnerabilities. Finally, we advocate for combinatorial approaches that couple metabolic rewiring with immunotherapies, proposing that disrupting amino acid dependencies in TAMs could dismantle the immunosuppressive TME.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


