Epac1缺失可减弱缺血性视网膜病变中突触神经胶质的病理激活并减轻视网膜神经退行性变
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
缺血性视网膜病变,如早产儿视网膜病变和增殖性糖尿病视网膜病变,是导致视力丧失的主要原因。病理性新生血管和神经元损伤都会导致这些情况下的视力障碍,但目前的治疗主要针对新生血管,并可能进一步损害神经元健康。目的环腺苷酸(cAMP)是一种公认的调节多种生理和病理过程的第二信使。由cAMP直接激活的交换蛋白(Epac)是最近发现的cAMP信号传导效应物。本研究旨在探讨Epac1在缺血视网膜病变中的作用,并通过小鼠氧诱导视网膜病变(OIR)模型阐明其潜在机制。方法将幼鼠从出生第7天(P7)至P12暴露于70%氧环境中,建立小鼠ir模型。采用H&;E染色、TUNEL染色及免疫染色检测视网膜结构及神经元存活情况。通过单细胞RNA测序(scRNA-seq)、免疫染色、qPCR、Western blot和近端结扎实验来研究Epac1参与视网膜病变的细胞和分子机制。结果Epac1基因缺失可显著保护视网膜神经元的丧失和减弱神经突触的激活,并可减少病理性新生血管和促进血管修复。scRNA-seq显示,在OIR期间,m本文章由计算机程序翻译,如有差异,请以英文原文为准。
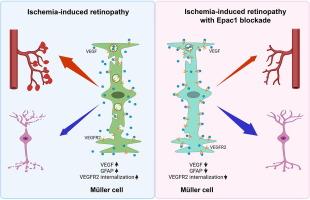
Epac1 deletion attenuates Müller glial pathological activation and mitigates retinal neurodegeneration in ischemia-induced retinopathy
Introduction
Ischemia-induced retinopathies, such as retinopathy of prematurity and proliferative diabetic retinopathy, are major causes of vision loss. Both pathological neovascularization and neuronal damage contribute to vision impairment in these conditions, yet current therapies primarily target neovascularization and may further compromise neuronal health.Objectives
Cyclic AMP (cAMP) is a widely recognized second messenger regulating diverse physiological and pathological processes. Exchange protein directly activated by cAMP (Epac) is a recently identified effector of cAMP signaling. This study aimed to investigate the role of Epac1 in ischemia-induced retinopathy and elucidate its underlying mechanisms using a mouse model of oxygen-induced retinopathy (OIR).Methods
A mouse OIR model was established by exposing pups to 70% oxygen from postnatal day 7 (P7) to P12. Retinal structure and neuronal survival were assessed by H&E staining, TUNEL assay and immunostaining. Single-cell RNA sequencing (scRNA-seq), immunostaining, qPCR, Western blot, and proximity ligation assay were used to investigate cellular and molecular mechanisms by which Epac1 contributes to retinal pathology in OIR.Results
Genetic deletion of Epac1 significantly protected against retinal neuronal loss and attenuated Müller glial activation in addition to reducing pathological neovascularization and promoting vascular repair. scRNA-seq revealed substantial transcriptomic alterations in Müller cells during OIR, including the emergence of reactive subclusters with distinct pathological roles, which were mitigated by Epac1 deletion. At the molecular level, Epac1 deletion restored ischemia-reduced VEGFR2 level in Müller cells during OIR, while Epac activation promoted VEGFR2 internalization and impaired VEGF signaling and its neuroprotective effects in primary Müller cells.Conclusion
These findings reveal a novel role for Epac1 in promoting retinal neurodegeneration in a mouse model of OIR through modulation of VEGF signaling in Müller cells. Targeting Epac1 may thus provide therapeutic benefit by preserving neuronal integrity in addition to its effects on vascular pathology.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


