从青藤衍生的共生真菌Acrocalymma cycadis中提取的嗜酸根烷A-I,嗜酸根烷型倍半萜类化合物。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究分离了9个先前未被描述的高氧羊角菌烯倍半萜类,命名为羊角菌烯A-I(1-9),以及3个已鉴定的类似物(10-12),这些类似物来源于青藤菌(Sinomenium acutum)。利用1D和2D NMR对代谢物进行了结构解析;HR-ESI-TOF-MS;单晶x射线衍射;和ECD谱计算。值得注意的是,粗亚麻红烷A(1)被鉴定为一种不寻常的氯化非亚麻红烷倍半萜类化合物,含有α,β-不饱和酮单元和烯醇片段。acroeremophanes F-H(6-8)被鉴定为罕见的糖基化的eremophane倍半萜类化合物,来源于共生真菌。体外细胞毒性实验结果表明,C-1取代的氯化紫芹烷倍半萜类具有明显的细胞毒性,其中红芹烷C(3)对HeLa和A549细胞具有较强的细胞毒性,IC50值分别为2.89和4.55 μM。细胞凋亡实验结果表明,化合物3主要诱导凋亡细胞死亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
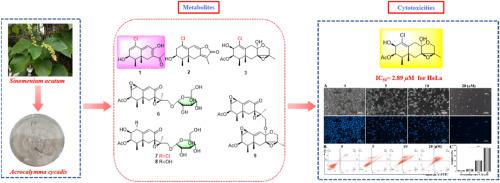
Acroeremophilanes A–I, eremophilane-type sesquiterpenoids from the Sinomenium acutum-derived symbiotic fungus Acrocalymma cycadis
This study separated nine previously undescribed highly oxygenated eremophilane sesquiterpenoids, designated as acroeremophilanes A–I (1–9), as well as three identified analogs (10–12), in the symbiotic fungus Acrocalymma cycadis derived from Sinomenium acutum. Structural elucidation of the metabolites was achieved using 1D and 2D NMR; HR-ESI-TOF-MS; single-crystal X-ray diffraction; and ECD spectra calculations. Notably, acroeremophilane A (1) was identified as an unusual chlorinated nor-eremophilane sesquiterpenoid incorporating an α,β-unsaturated ketone unit with an enol fragment. Acroeremophilanes F–H (6–8) were characterized as rare glycosylated eremophilane sesquiterpenoids derived from the symbiotic fungus. The in vitro cytotoxicities showed that C-1 substituted chlorinated eremophilane sesquiterpenoids displayed obvious cytotoxicity, in which acroeremophilane C (3) exhibited potent cytotoxicity to HeLa and A549 cells, and IC50 values were 2.89 and 4.55 μM, separately. The results of the apoptosis assays indicated that compound 3 primarily induces apoptotic cell death.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


