开发儿童药品与常用给药工具(果汁、苹果酱、酸奶和布丁)相容性测试的标准工具箱。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-15
DOI:10.1016/j.ejpb.2025.114868
引用次数: 0
摘要
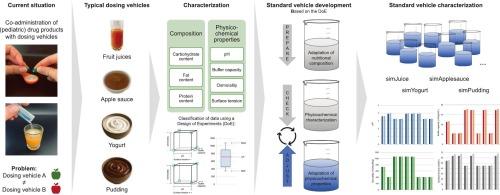
口服药物与给药载体共同给药是儿科给药的常见做法。目前的工作重点是一套标准车辆的发展,反映了四种不同的车辆类型通常用于儿科药物配方管理的关键特点。目的是使儿童剂型与单个载体类型的相容性评估合理化和标准化,从而有助于更好地评估这种给药途径的风险。为了制定标准车辆,对果汁、苹果酱、酸奶和布丁的理化性质进行了全面的表征。表征结果作为一组标准车辆的目标值,该标准车辆已成功开发并遵循实验方法设计。这些标准车辆代表了为全球使用而设计的标准化车辆工具箱的第一个和核心组件。它们的使用将使制药开发商和监管当局能够深入了解儿科药物与这些给药工具的兼容性。在通过与原始载体的比较研究验证之前,该工具箱有望成为评估儿科配方与液体或半固体载体结合的安全性和可行性的宝贵资源,从而支持药物开发中更明智的决策。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Towards the development of a standard toolbox for compatibility testing of pediatric drug products with common dosing vehicles − Fruit juice, apple sauce, yogurt, and pudding
Co-administration of oral drug products with dosing vehicles is common practice in pediatric drug administration. The present work focused on the development of a set of standard vehicles that reflects the key characteristics of four different vehicle types commonly used in the administration of pediatric drug formulations. The aim was to rationalize and standardize the compatibility assessment of pediatric dosage forms with individual vehicle types and thereby contribute to a better risk assessment regarding this route of administration. In order to develop the standard vehicles, a comprehensive number of fruit juices, apple sauces, yogurts, and puddings were characterized with regard to their physicochemical properties. The characterization results served as target values for a set of standard vehicles which was successfully developed and followed a design of experiments approach. These standard vehicles represent the first and central components of a standardized vehicle toolbox designed for global use. Their use will enable pharmaceutical developers and regulatory authorities to gain insight into the compatibility of pediatric medicines with these dosing vehicles. Pending validation through comparative studies with the original vehicles, for which data are forthcoming, the toolbox is expected to serve as a valuable resource for assessing the safety and feasibility of combining pediatric formulations with liquid or semi-solid vehicles, thereby supporting more informed decision-making in drug development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


