IFN-γ通过上调TLR3在胆管癌细胞中增强Poly(I:C)诱导的坏死坏死和免疫原性细胞死亡。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
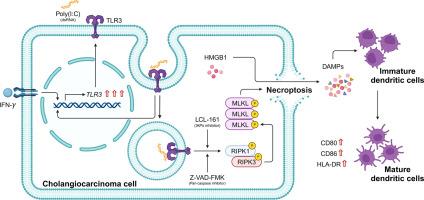
胆管癌(CCA)是一种起源于胆管的异质性肿瘤,由于有效治疗方案有限,预后不良,死亡率高。尽管有希望,但CCA的结缔组织增生肿瘤微环境阻碍了免疫治疗,该微环境以其炎症和免疫抑制特征而闻名,通常被称为“冷肿瘤”。先前,我们报道了Poly(I:C),一种TLR3激动剂,与一种Smac模拟物,一种IAP拮抗剂协同作用,当caspase活性被阻断时触发坏死坏死。在本研究中,我们旨在评估临床前模型中基于Poly(I:C)的坏死性上睑下垂免疫治疗的合理联合策略。对公开CCA患者数据集的转录组学分析显示,TLR3的低表达,伴随着IFNG或IRF1的低表达,与较差的预后显著相关。这些发现强调了IFN-γ/IRF1/TLR3轴的临床相关性,并支持了针对该途径的治疗方法的开发。我们进一步发现IFN-γ处理增加了TLR3的表达。重要的是,我们证明了当IAPs和caspase被Smac模拟物和泛caspase抑制剂Z-VAD-FMK抑制时,IFN-γ可以增强Poly(I:C)诱导的细胞坏死和免疫原性细胞死亡(ICD)。CU-CPT 4a是一种高选择性TLR3抑制剂,部分减少了这种细胞死亡。这表明该效应部分是通过TLR3介导的。联合作用依赖于RIPK1/RIPK3/ mlkl诱导的坏死下垂。此外,濒死细胞释放HMGB1 (ICD的标志物),其条件培养基促进树突状细胞成熟(CD80, CD86, HLA-DR),这对抗原呈递和t细胞启动至关重要。从个性化医学的角度来看,TLR3、IFNG和IRF1的表达水平可以作为预测性生物标志物,指导患者选择基于TLR3激动剂的治疗方法。这些发现可能会促进TLR3配体,Poly(I:C)为基础的坏死性上睑癌免疫治疗,单独或与免疫检查点抑制剂,用于CCA和可能的其他癌症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
IFN-γ enhances Poly(I:C)-induced necroptosis and immunogenic cell death via TLR3 upregulation in cholangiocarcinoma cells
Cholangiocarcinoma (CCA), a heterogeneous tumor arising in the bile ducts, is associated with unfavorable prognosis and high mortality rates due to limited effective treatment options. Though promising, immunotherapy is hindered by CCA's desmoplastic tumor microenvironment, known for its inflammatory and immunosuppressive characteristics, often termed a “cold tumor”. Previously, we reported that Poly(I:C), a TLR3 agonist, synergizes with a Smac mimetic, an IAP antagonist, to trigger necroptosis when caspase activity is blocked. In this study, we aimed to evaluate rational combination strategies to advance Poly(I:C)-based necroptosis immunotherapy in preclinical models. Transcriptomic analysis of public CCA patient datasets revealed that low expression of TLR3, accompanied by low IFNG or IRF1 expression, is significantly associated with poorer prognosis. These findings highlight the clinical relevance of the IFN-γ/IRF1/TLR3 axis and support the development of therapies targeting this pathway. We further found that IFN-γ treatment increases TLR3 expression. Importantly, we demonstrated that IFN-γ could enhance Poly(I:C)-induced necroptosis and immunogenic cell death (ICD) when IAPs and caspases are inhibited by Smac mimetic and the pan-caspase inhibitor Z-VAD-FMK in human CCA cell lines. CU-CPT 4a, a highly selective TLR3 inhibitor, partially reduced this cell death. This indicates that the effect is mediated in part through TLR3. The combined effects were shown to depend on RIPK1/RIPK3/MLKL-induced necroptosis. Additionally, the dying cells released HMGB1, a marker of ICD, and their conditioned medium promoted dendritic cell maturation (CD80, CD86, HLA-DR), which is critical for antigen presentation and T-cell priming. From a personalized medicine perspective, TLR3, IFNG, and IRF1 expression levels may serve as predictive biomarkers to guide patient selection for TLR3 agonist-based therapies. These findings could advance TLR3 ligand, Poly(I:C)-based necroptosis cancer immunotherapy, alone or with immune checkpoint inhibitors, for CCA and possibly other cancers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


