烯与亲碳亲烯试剂反应的最新进展
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
烯反应是四电子“烯”组分与两个电子亲烯官能团之间的分子间或分子内加成反应,其中两个π键和一个C-H(或金属- c) σ键被重排成一个π键和两个σ键。新形成的σ-键一个是碳-碳键,另一个是重排的碳-氢(或金属-碳)键。这种转化可以在一个步骤中形成结构和功能复杂的烯产品。最初,烯反应被发现为协调的、逐步的周环基本过程。然而,通过实验和计算研究,以及广泛的经验方法工作,不断增加的机理见解,为涉及亲碳试剂的过多的烯型反应铺平了道路。自2012年以来,已经建立的过渡金属催化的烯反应变体得到了显着扩展。此外,高活性的芳基中间体——在室温下由氟诱导的邻硅基芳基三氟酯消除原位生成,或由1,3-二炔与炔(分子间和分子内)的六氢- diels - alder反应生成——在新型基因型序列中越来越多地应用。丙炔反应也获得了关注,特别是因为产生的烯-烯在设计导致复杂多环融合结构的多米诺骨牌序列中作为非常有价值的中间体。最后,在聚合物化学中实施烯反应来合成功能化聚合物和可再生的不饱和原料已经成为使用纯石化基底物的有希望的替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
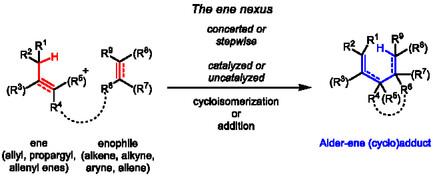
Recent Advances in Ene Reactions with Carbon Enophiles
Ene reactions are inter- or intramolecular addition reactions between a four-electron "ene" component and a two-electron enophile functionality, in which two π-bonds and a C–H (or metal–C) σ-bond are rearranged into one π-bond and two σ-bonds. One of the newly formed σ-bonds is a carbon–carbon bond, while the other is the rearranged a C–H (or metal–C) bond. This transformation enables the formation of structurally and functionally complex ene products in a single step. Originally, ene reactions were discovered as concerted and stepwise pericyclic elementary processes. However, increasing mechanistic insight—gained through both experimental and computational studies—along with extensive empirical methodological work, has paved the way to a plethora of ene-type reactions involving carbon enophiles. Since 2012, the already well-established transition metal-catalyzed variants of ene reactions have significantly expanded. Moreover, highly reactive aryne intermediates—generated in situ either by fluoride induced elimination from ortho-silyl aryltriflates at room temperature, or by hexadehydro-Diels–Alder reaction of a 1,3-diyne with an alkyne (both inter- and intramolecularly)—find increasing application in novel ene-type sequences. Propargyl ene reactions also gain traction, particularly because the resulting ene-allenes serve as highly valuable intermediates in the design of domino sequences that lead to complex polycyclic fused structures. Finally, the implementation of ene reactions in polymer chemistry for the synthesis of functionalized polymers and renewable unsaturated raw materials has emerged as a promising alternative to the use of purely petrochemistry based substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


