可见光催化从硫烷选择性合成1,2-或1,3-二硫烷的实验和理论研究
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
与其他含硫杂环不同,功能化二硫烷的潜在应用由于其合成具有挑战性,迄今为止尚未得到充分的探索。为了解决这个问题,我们在此利用直接获得功能化硫烷的途径,并描述了利用硫羰基底物将后者选择性地扩大到1,2-或1,3-二硫烷的光化学环,这些底物是通过诺里希II型裂解反应原位生成的。通过光催化不同取代的硫烷和各种硫羰基之间的多米诺骨牌序列获得的二硫烷文库揭示了区域选择性规则:激发的硫酮只产生1,2-二硫烷,而激发的硫醛选择性地产生1,3-二硫烷,其中1,2-二硫烷是其区域异构体的前体。通过Norrish-II型硫代羰基产物与不同的烯烃伙伴通过thia-Paternò_Büchi反应得到硫代烷中间体,从而得到1,2-二硫代烷。这些实验结果与时间依赖的密度泛函理论计算相补充,支持了复杂的化学途径,使观察到的区域选择性合理化,并进一步证实了与肽中二硫桥的光解有关的机制建议。本文章由计算机程序翻译,如有差异,请以英文原文为准。
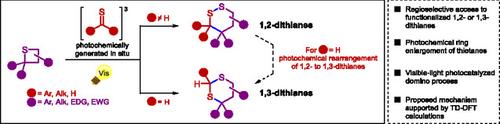
Visible-Light Photocatalyzed Selective Synthesis of 1,2- or 1,3-Dithianes From Thietanes: An Experimental and Theoretical Study
Unlike other sulfur-containing heterocycles, the potential applications of functionalized dithianes have been less explored to date due to their challenging synthesis. To address this issue, we herein leverage the straightforward access to functionalized thietanes and describe a selective photochemical ring enlargement of the latter to 1,2- or 1,3-dithianes using thiocarbonyl substrates, which are in situ generated via a Norrish-type II fragmentation reaction. The library of dithianes obtained by this photocatalyzed domino sequence between diversely substituted thietanes and various thiocarbonyls revealed regioselectivity rules: excited thioketones lead exclusively to 1,2-dithianes, while excited thioaldehydes selectively yield 1,3-dithianes, where 1,2-dithianes serve as precursors to their regioisomers. The ring expansion reaction leading to 1,2-dithianes was also integrated into a triple photochemical cascade, where the thietane intermediates were obtained via a thia-Paternò_Büchi reaction between Norrish-II thiocarbonyl products and various alkene partners. These experimental results were complemented with time-dependent density functional theory calculations, which support the complex chemical pathway, rationalize the observed regioselectivity, and further substantiate mechanistic proposals related to the photolysis of disulfide bridges in peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


