用TiP2O7选择性电化学萃取盐水中的Li+
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于对锂离子电池(LIBs)的需求不断增长,对锂的需求不断增加。目前,超过50%的锂来自富含Li+的卤水矿床。此外,锂离子电池制造业产生的废水中含有Li+和其他阳离子的混合。因此,从各种天然和工业含Li+溶液中选择性提取Li+的方法的发展是确保可持续和环保的Li供应链的关键。这项研究报道了TiP2O7 (TPO)作为一种有前途的候选材料,可以从含有Na+、K+、Mg2+和Ca2+离子浓度高于Li+的混合物的溶液中选择性地提取和回收Li+。此外,TPO还展示了从玻利维亚Uyuni盐水中提取Li+并将其回收为Li2CO3的能力。它比LiFePO4和LiMn2O4这两种之前研究过的从盐水中选择性提取Li+的候选材料具有独特的优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
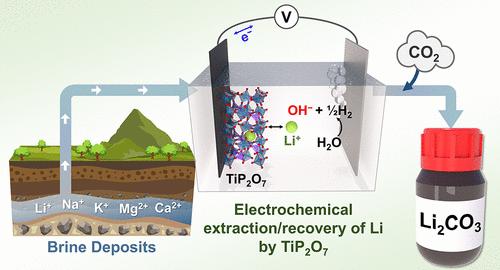
Selective Electrochemical Li+ Extraction from Brines Using TiP2O7
The demand for Li keeps increasing due to the growing need for Li-ion batteries (LIBs). More than 50% of Li is currently obtained from Li+-rich brine deposits. Also, the LIB manufacturing industry generates wastewater containing Li+ mixed with other cations. Thus, the development of methods to selectively extract Li+ from various natural and industrial Li+-containing solutions are critically needed to ensure a sustainable and environmentally benign Li supply chain. This study reports TiP2O7 (TPO) as a promising candidate for selective electrochemical Li+ extraction and recovery from solutions containing a mixture of cations where Na+, K+, Mg2+, and Ca2+ are more concentrated than Li+. In addition, TPO’s ability to extract Li+ from a simulated brine mimicking the Uyuni Brine in Bolivia and recovering it as Li2CO3 is demonstrated. Its unique advantage over LiFePO4 and LiMn2O4, two candidates previously investigated for selective Li+ extraction from brines, is also discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


