含杂化膦配体的多孔有机聚合物上单原子Rh位点上空间位阻烯烃的区域选择性氢甲酰化
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
开发能够对空间位阻烯烃进行高效和区域选择性氢甲酰化的多相催化剂仍然是一个重大挑战。在这项工作中,我们开发了一种稳定和活跃的Rh单原子催化剂(Rh@POP-X),锚定在具有定制孔隙率和高比表面积的杂化膦功能化多孔有机聚合物(POP)上,允许立体受阻二异丁烯(DIB)的氢甲酰化。包括固态NMR, AC HAADF-STEM, XPS,原位CO-DRIFTS, XAS在内的综合表征证实了交联聚合物网络中由膦配体协调的Rh位点的原子分散。最佳催化剂Rh@POP-2表现出优异的性能,达到 >;98 %的转化率,对线性异壬醛具有优异的区域选择性。值得注意的是,Rh@POP-2催化剂表现出高稳定性,在连续10次反应循环中保持其催化性能而没有明显的失活。该催化体系的多功能性进一步证明了其广泛的底物适应性,使各种烯烃(包括线性烯烃、末端烯烃、芳香烯烃和位阻烯烃)的氢甲酰化有效,从而生成相应的C1+醛。这项工作为设计强大的单原子催化剂来挑战立体受阻烯烃的氢甲酰化提供了一般策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
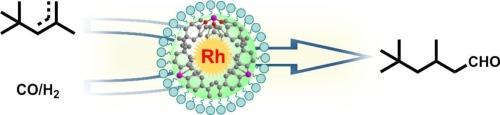
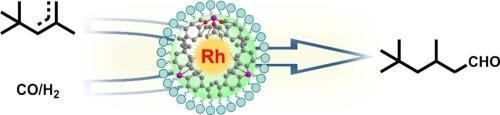
Regioselective hydroformylation of sterically hindered olefins over single-atom Rh sites on porous organic polymer incorporating hybrid phosphine ligands
Developing heterogeneous catalysts capable of efficient and regioselective hydroformylation of sterically hindered alkenes remains a significant challenge. In this work, we develop a stable and active Rh single-atom catalyst (Rh@POP-X) anchored on a hybrid phosphine-functionalized porous organic polymer (POP) with tailored porosity and high specific surface area, allowing for hydroformylation of sterically hindered diisobutylene (DIB). Comprehensive characterizations including solid-state NMR, AC HAADF-STEM, XPS, in-situ CO-DRIFTS, XAS confirm the atomic dispersion of Rh sites coordinated by phosphine ligands within the crosslinked polymeric network. The optimal catalyst Rh@POP-2 exhibits exceptional performance, achieving >98 % conversion with excellent regioselectivity toward linear isononanal. Remarkably, the Rh@POP-2 catalyst demonstrates high stability, maintaining its catalytic performance over 10 times successive reaction cycles without significant deactivation. The versatility of this catalytic system is further demonstrated by its broad substrate adaptability, enabling efficient hydroformylation of various olefins, including linear, terminal, aromatic, and sterically hindered olefins, to yield their corresponding C1+ aldehydes. This work provides a general strategy for designing robust single-atom catalysts for challenging hydroformylation of sterically hindered olefins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


