在与胺的CO2还原偶联反应中,阳离子铟配合物使H2分压减半
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
铟催化的均相加氢仅限于亚胺加氢,因为In(C6F5)3的热和水解稳定性较低,In(C6F5)3是迄今为止唯一用于加氢化学的铟刘易斯酸。在这项研究中,我们提出了[In(terpy)Cl2]+阳离子作为刘易斯酸催化剂。在H2(50 bar)和CO2(4 bar)压力下,[In(terpy)Cl2]+在H2(50 bar)和CO2(4 bar)压力下有效催化了CO2与胺的还原偶联,具有H2活化的最佳空位和高达579 kJ mol−1的氢化物离子亲和力,XRD和NMR结构证实了这一点。通过在所有其他主基团催化体系的一半分压下与H2催化CO2与胺的还原偶联,阳离子铟配合物为此类反应设定了新的基准。因此,本研究不仅扩大了铟基催化亚胺加氢的范围,而且突出了配体负载的铟路易斯酸作为小分子活化催化体系的潜力本文章由计算机程序翻译,如有差异,请以英文原文为准。

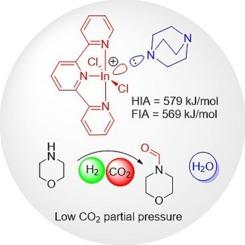
Cationic indium complexes halve H2 partial pressure in CO2 reductive coupling reactions with amines
Indium-catalysed homogeneous hydrogenations are limited to imine hydrogenation due to the low thermal and hydrolytic stability of In(C6F5)3, the only indium Lewis acid used thus far for hydrogenation chemistry. In this study, we present [In(terpy)Cl2]+ cations as Lewis acid catalysts. With an optimal vacant site for H2 activation and a hydride ion affinity of up to 579 kJ mol−1, [In(terpy)Cl2]+ effectively catalysed CO2 reductive coupling with amines under H2 (50 bar) and CO2 (4 bar) pressure, as structurally confirmed by XRD and NMR spectroscopy. By catalysing CO2 reductive coupling with amines at half the partial pressure of all other main-group catalytic systems with H2, cationic indium complexes set a new benchmark for such reactions. Therefore, this study not only expands the scope of indium-based catalysis beyond imine hydrogenation but also highlights the potential of ligand-supported indium Lewis acids as catalytic systems for small-molecule activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


