线粒体靶向杂交纳米囊泡触发免疫原性细胞死亡并重塑肿瘤中性粒细胞,增强结直肠癌免疫治疗
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
癌症免疫疗法因其恢复免疫反应以有效消除癌症的能力而受到广泛关注。在免疫细胞中,中性粒细胞在癌症中起着双刃剑的作用,选择性激活有益的中性粒细胞群和靶向抑制促瘤中性粒细胞在癌症治疗中显示出巨大的潜力。在这里,我们开发了一种能够靶向结直肠癌线粒体(MNeutrosome)的中性粒细胞模拟纳米载体(Neutrosome),它由活性中性粒细胞膜与线粒体靶向脂质体杂交组成,脂质体装载β-Lapachone (Liposome@Lapa),被认为可以诱导免疫原性细胞死亡(ICD)并调节免疫微环境。我们的研究结果表明,MNeutrosome@Lapa选择性地在结直肠癌线粒体中积累并激活醌氧化还原酶-1 (NQO1),导致活性氧(ROS)的产生和随后的癌细胞凋亡。值得注意的是,卸载的MNeutrosome被证明可以减少中性粒细胞对肿瘤组织的浸润,而MNeutrosome@Lapa将肿瘤相关中性粒细胞(tan)从促肿瘤的N2表型重编程为抗肿瘤的N1表型,进一步促进免疫调节。总体而言,MNeutrosome@Lapa表现出良好的生物相容性和显著的治疗效果,是CRC治疗的一个有前景的治疗平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。

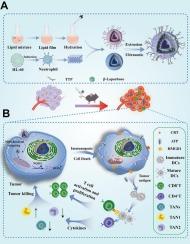
Mitochondria-targeted hybrid nanovesicles trigger immunogenic cell death and remodel tumor neutrophils for enhanced colorectal cancer immunotherapy
Cancer immunotherapy has garnered significant attention for its ability to reinstate immune responses to eliminate cancer effectively. Among immune cells, neutrophils play a dual-edged sword role in cancer, and the selective activation of beneficial neutrophil populations and targeted inhibition of tumor-promoting neutrophils have shown significant potential in cancer therapy. Here, we developed a neutrophil mimic nanovehicle (Neutrosome) capable of targeting colorectal cancer mitochondria (MNeutrosome), which consists of active neutrophil membranes hybridized with mitochondria-targeting liposome loaded with β-Lapachone (Liposome@Lapa), which is proposed to induce immunogenic cell death (ICD) and modulate the immune microenvironment. Our findings demonstrate that MNeutrosome@Lapa selectively accumulates in colorectal cancer mitochondria and activates quinone oxidoreductase-1 (NQO1), leading to robust reactive oxygen species (ROS) production and subsequent cancer cell apoptosis. Notably, the unloaded MNeutrosome was shown to reduce neutrophil infiltration into tumor tissue, whereas MNeutrosome@Lapa reprogrammed tumor-associated neutrophils (TANs) from a pro-tumor N2 phenotype to an anti-tumor N1 phenotype, further contributing to immune modulation. Overall, MNeutrosome@Lapa demonstrated excellent biocompatibility and significant therapeutic efficacy and represents a promising therapeutic platform for CRC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


