从医药产品中使用的橡胶塞系统中获得可提取和可浸出物质的不同提取方法的评价和特性。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-14
DOI:10.1016/j.ejpb.2025.114844
引用次数: 0
摘要
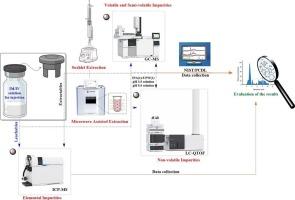
溴丁基橡胶塞广泛应用于许多不同药品的初级包装系统中。然而,添加不同的添加剂以提供这些合成弹性体塞的现有性能。最重要的添加剂是固化剂、活化剂、增塑剂、填料、抗氧化剂和促进剂。然而,这些成分不是共价键合到聚合物链上,并且在其保质期内有可能从橡胶塞材料中浸出到药品中。因此,由于它们可能会对产品的安全性、有效性和稳定性产生不利影响,因此应该通过可提取的研究来识别和监测它们。此外,美国食品和药物管理局(FDA)和欧洲药品管理局(EMA)等监管机构要求提交可提取物和可浸出物(E&L)信息,以检查风险。为此目的,在研究范围内检查了源自溴丁基橡胶塞的E&L。采用两种不同的样品制备方法提取最高水平的挥发性、半挥发性和非挥发性成分。微波辅助提取法与传统索氏提取法进行了比较,微波辅助提取法比索氏提取法更快,溶剂消耗更少,并且与包装基质的接触时间更长,持续时间更长。结果表明,微波辅助提取法得到的成分数量较高。此外,使用不同的溶剂环境(IPA + 水,pH 3.5溶液和pH 9.5溶液)将基质暴露在极端条件下以获得非挥发性成分。利用气相色谱-质谱联用(GC-MS)和液相色谱四极杆飞行时间质谱联用(LC-Q-TOF)分析仪器的数据库对提取得到的可提取成分进行鉴定。元素杂质采用电感耦合等离子体质谱法(ICP-MS)定量测定,并在可能的添加剂范围内进行评价。此外,在可浸出性研究的范围内,对容器盖系统中使用溴丁基橡胶塞的六种不同药品配方进行了调查。结果表明,所提出的方法在不同治疗产品的E&L分析中是成功的。此外,它适用于不同初级包装产品的分析,以确保产品质量和患者安全,同时也为文献和制药行业提供了一种新的盈亏分析方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation and characterization of different extraction methods for obtaining extractable and leachable materials from rubber stopper systems used in pharmaceutical products
Bromobutyl rubber stoppers are widely used in the primary packaging systems of many different drug products. However, different additives are added to provide the existing properties of these synthetic elastomeric stoppers. The most important additives are curing agents, activators, plasticizers, fillers, antioxidants and accelerators. However, these components are not covalently bonded to the polymer chains and have the potential to leach from the rubber stopper material to the drug product during its shelf life. Therefore, since they may adversely affect the product’s safety, efficacy and stability, they should be identified and monitored through extractable studies. In addition, regulatory authorities such as the US Food and Drug Administration (US FDA) and the European Medicines Agency (EMA) require the submission of Extractables and Leachables (E&L) information to examine the risks. For this purpose, E&L originating from bromobutyl rubber stoppers was examined within the scope of the study. Two different sample preparation methods were used to extract volatile, semi-volatile and non-volatile components at the highest level. The microwave assisted extraction methods, which are relatively widely used and provide faster and less solvent consumption than Soxhlet and also provide longer and continuous contact with the packaging matrix, were compared with the conventional Soxhlet extraction method. The results show that the number of components obtained by microwave assisted extraction method is higher. In addition, different solvent environments (IPA + water, pH 3.5 solution and pH 9.5 solution) were used to expose the matrix to extreme conditions to obtain non-volatile components. The extractable components obtained were identified by using the library databases of the relevant gas chromatography-Mass Spectrometry (GC–MS) and liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF) analytical devices. Elemental impurities were quantitatively determined by inductively coupled plasma mass spectrometry (ICP-MS) and evaluated within the scope of possible additives. In addition, six different drug product formulations using bromobutyl rubber stoppers in container-lid systems were investigated within the scope of leachable studies. The results show that the presented methodology is successful in the E&L analysis of different therapeutic products. Moreover, it is adaptable to the analysis of different primary packaging products to ensure product quality and patient safety, while also offering a new approach to E&L analysis for both the literature and the pharmaceutical industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


