靶向溶菌酶2在心内膜通过调节远程损伤信号促进快速恢复
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
成年哺乳动物的心脏是不可再生的,大多数关于心肌梗死(MI)后修复和潜在再生的研究都集中在心肌细胞(CM)增殖和梗死区。在这里,我们观察到溶菌酶2 (Lyz2)在损伤小鼠心脏的局部损伤部位和远处区域异常高表达,在非再生心脏的心内膜细胞中持续表达明显。虽然传统上被认为是髓系标志物,但我们证明LYZ2是一种损伤特异性的、溶酶体降解能力的正调节因子,介导细胞外基质的致病性降解。在小鼠和人心肌内膜实验模型中,我们观察到破坏LYZ2/LYZ功能对CMs具有抗凋亡作用。利用这些见解,我们表明Lyz2敲除(KO)和溶酶体降解的药理抑制都能使受伤的非再生心脏快速功能恢复。因此,靶向非cm细胞类型的远程损伤反应可迅速促进非再生心脏的心肌梗死后恢复。本文章由计算机程序翻译,如有差异,请以英文原文为准。
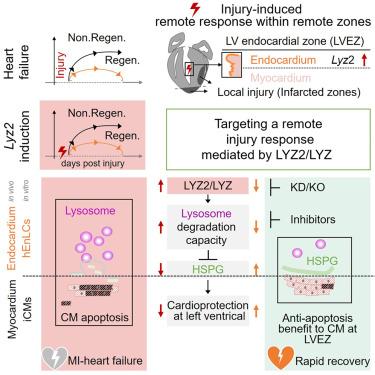
Targeting lysozyme 2 in endocardium promotes rapid recovery by modulating remote injury signals
Adult mammalian hearts are non-regenerative, and a majority of studies examining repair and potential regeneration post-myocardial infarction (MI) have focused on cardiomyocyte (CM) proliferation and infarcted zones. Here, we observed aberrantly high expression of lysozyme 2 (Lyz2) in injured mouse hearts at both local injury sites and at remote zones, with sustained Lyz2 expression conspicuous in endocardial cells of non-regenerative hearts. Although traditionally conceptualized as a myeloid marker, we demonstrate that LYZ2 functions as an injury-specific, positive regulator of lysosomal degradation capacity that mediates pathogenic degradation of the extracellular matrix. We observed an anti-apoptotic benefit to CMs upon disrupting LYZ2/LYZ function in mice and in a human endomyocardium experimental model. Harnessing these insights, we show that both Lyz2 knockout (KO) and pharmacological inhibition of lysosomal degradation confer rapid functional recovery in injured non-regenerative hearts. Thus, targeting a remote injury response in a non-CM cell type rapidly promotes post-MI recovery of non-regenerative hearts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


