空间模式的肾脏组装体概括了祖细胞的自组装,并实现了高保真的体内疾病建模
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
目前的肾脏类器官不能概括肾脏复杂的空间模式和功能,限制了它们的应用。人体肾脏由100万个肾单位组成,这些肾单位来源于肾单位祖细胞,它们连接到一个由输尿管祖细胞衍生的收集系统。在这里,我们建立了空间组织的小鼠和人类肾脏祖细胞组装体(KPA)模型,其中肾单位经历广泛的发育并融合到一个集中的收集系统,再现了在体内观察到的肾脏祖细胞自组装过程。在体外和体内,KPAs显著改善了细胞的复杂性和成熟度,并表现出主要肾脏功能的几个方面。用基因组编辑的、体内培养的人kpa模拟人类常染色体显性多囊肾病(ADPKD),再现了该疾病的囊性表型、分子和细胞特征,并突出了囊肿上皮、间质和巨噬细胞之间的串扰。KPA平台为高保真疾病建模开辟了新的途径,为肾脏再生医学奠定了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
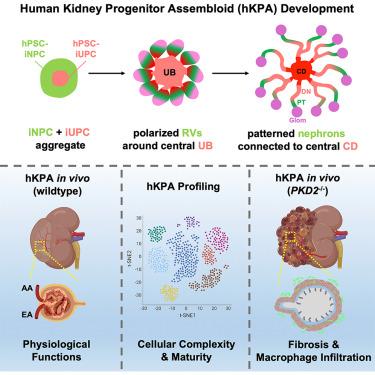
Spatially patterned kidney assembloids recapitulate progenitor self-assembly and enable high-fidelity in vivo disease modeling
Current kidney organoids do not recapitulate the kidney’s complex spatial patterning and function, limiting their applications. The human kidney comprises one million nephrons, derived from nephron progenitor cells, that connect to an arborized ureteric progenitor cell-derived collecting system. Here, we develop spatially organized mouse and human kidney progenitor assembloid (KPA) models in which the nephrons undergo extensive development and fuse to a centrally located collecting system, recapitulating kidney progenitor self-assembly processes observed in vivo. KPAs show dramatically improved cellular complexity and maturity and exhibit several aspects of major kidney functions in vitro and in vivo. Modeling human autosomal dominant polycystic kidney disease (ADPKD) with genome-edited, in vivo-grown human KPAs recapitulated the cystic phenotype and the molecular and cellular hallmarks of the disease and highlighted the crosstalk among cyst epithelium, stroma, and macrophages. The KPA platform opens new avenues for high-fidelity disease modeling and lays a strong foundation for kidney regenerative medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


