烯烃电化学驱动的溴氯化反应
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
有机卤化物在医药、农用化学品和材料合成中起着至关重要的作用。传统的烯烃二卤化使用活性卤素试剂,而我们开发了一种选择性溴氯化的电化学策略。这种方法可以在温和的条件下实现精确的卤化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
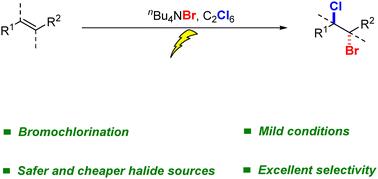
Electrochemically driven bromochlorination of alkenes
Organic halides are vital in the synthesis of pharmaceuticals, agrochemicals, and materials. While conventional alkene dihalogenation uses active halogen reagents, we developed an electrochemical strategy for selective bromochlorination. This approach enables precise halogenation under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


