微波辅助一锅绿色合成,ADMET分析,DFT和分子对接的新型3-氰基-2-吡啶酮衍生物作为双重EGFR和细胞因子(TNF-α, IL-6)抑制剂
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
表皮生长因子受体(EGFR)对癌细胞的生长、存活和耐药性至关重要。炎症因子如白细胞介素-6 (IL-6)和肿瘤坏死因子-α (TNF-α)也有助于肿瘤生长和逃避免疫系统。开发同时打击癌症和炎症途径的多靶点抑制剂具有很强的治疗潜力。本研究旨在设计、创建和测试一系列新的吡啶酮衍生物,这些衍生物通过抑制IL-6和TNF-α,作为EGFR信号通路的双重抑制剂,具有抗炎作用。我们合成了一组新的吡喃酮衍生物并进行了结构表征。我们使用计算机ADMET分析检查了它们的物理化学和药代动力学性质。我们通过MTT试验评估了化合物的抗癌活性,测试了MCF-7(乳腺癌)和A549(肺癌)细胞系的活力和细胞毒性。我们进一步评估了所选化合物的EGFR抑制作用和细胞因子抑制作用。流式细胞术(Annexin V/PI染色)检测细胞凋亡诱导情况。在对接研究中,一些新化合物显示出与EGFR的高结合亲和力。其中,化合物5h对A549和MCF7细胞均表现出较强的抗肿瘤活性,IC50分别为9.2 μM和8.2 μM,并能显著降低A549细胞的IL-6和TNF-α水平,ADMET谱也较好。流式细胞术证实细胞凋亡诱导呈剂量依赖性。我们进行了分子对接,以评估对EGFR和相关炎症靶点的结合亲和力。DFT计算通过HOMO/LUMO和分子静电势(ESP)研究来分析分子反应性。ESP图谱分析支持理论描述符,并证实化合物5h是合成衍生物中生物活性最高的候选化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。

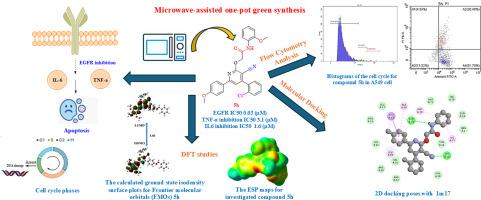
Microwave-assisted one-pot green synthesis, ADMET profiling, DFT, and molecular docking of novel 3-cyano-2-pyridone derivatives as dual EGFR and cytokine (TNF-α, IL-6) inhibitors
Epidermal Growth Factor Receptor (EGFR) is crucial for cancer cell growth, survival, and resistance. Inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) also help tumors grow and evade the immune system. Developing multi-targeted inhibitors that hit both cancerous and inflammatory pathways has strong therapeutic potential. This study aims to design, create, and test novel series of pyridone-based derivatives serving as dual inhibitors of EGFR signaling pathways with anti-inflammatory effects by suppressing IL-6 and TNF-α. We synthesized and structurally characterized a set of novel pyridone derivatives. We checked their physicochemical and pharmacokinetic properties using in silico ADMET profiling. We evaluated the anticancer activity of the compounds through the MTT assay, testing viability and cytotoxicity in MCF-7 (breast) and A549 (lung) cancer cell lines. We further assessed selected compounds for their EGFR inhibitory effects and cytokine suppression. We conducted flow cytometry (Annexin V/PI staining) to evaluate apoptosis induction. Some of the novel compounds showed high binding affinity to EGFR in docking studies. Among these, compound 5h showed potent anticancer activity on both tested cell lines A549 and MCF7 with an IC50 of 9.2 μM and 8.2 μM respectively also it significantly reduced IL-6 and TNF-α levels in A549 cells, also showed promising ADMET profiles. Flow cytometry confirmed apoptosis induction in a dose-dependent manner. We performed molecular docking to evaluate the binding affinity against EGFR and related inflammatory targets. DFT calculations were performed to analyze molecular reactivity through HOMO/LUMO and molecular electrostatic potential (ESP) studies. ESP map analysis supports theoretical descriptors and confirms that compound 5h is the most biologically active candidate among the synthesized derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


