c -28改性吡唑-融合白桦酸衍生物抗骨质疏松的合成及生物学评价
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
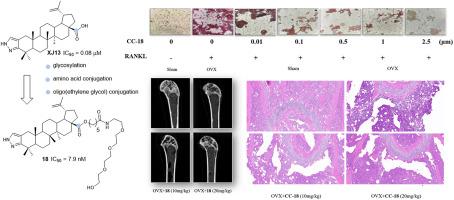
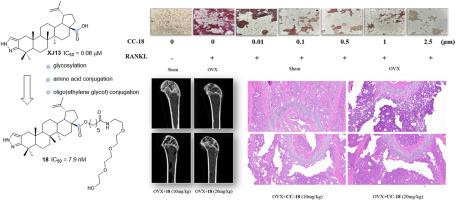
设计、合成了一系列新的C-28氨基酸/酰胺缀合物和低聚乙二醇修饰的C-2、C-3吡唑-融合白桦酸衍生物,并评估了它们对rankl诱导的破骨细胞生成的抑制作用。其中,含有C-28寡聚(乙二醇)氨基酰胺酯键的化合物18表现出最有效的抑制活性。结果表明,对rankl诱导的RAW264.7细胞破骨细胞生成的IC50为7.96 nM,与XJ13 (IC50 = 0.08 μM)相比,效力提高了10倍,在0.01 μM处达到50%的抑制作用。重要的是,这些化合物对rankl诱导的破骨细胞分化的抑制作用并非归因于细胞毒性,正如化合物18在10 μM下的最小细胞毒性所证明的那样。机制研究表明,化合物18可以剂量依赖性地抑制破骨细胞标记基因(TRAP、CTSK)和蛋白(c-fos、MMP-9)的表达。此外,在卵巢切除(OVX)小鼠模型中,化合物18(10和20 mg/kg,腹腔注射)通过改善关键微ct参数和降低血清骨吸收标志物(CTx)剂量依赖性地预防骨丢失。总的来说,化合物18是治疗rankl驱动的骨质疏松症的一个非常有希望的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluation of C-28-modified pyrazole-fused betulinic acid derivatives as potent antiosteoporosis agents
A series of novel C-28 amino acid/amide conjugates and oligo(ethylene glycol)-modified C-2, C-3 pyrazole-fused betulinic acid derivatives were designed, synthesized, and evaluated for their inhibitory effects on RANKL-induced osteoclastogenesis. Among these, compound 18, bearing a C-28 oligo(ethylene glycol) amino amide ester linkage, exhibited the most potent inhibitory activity. It demonstrated an IC50 of 7.96 nM against RANKL-induced osteoclastogenesis in RAW264.7 cells, representing a 10-fold increase in potency compared to the XJ13 (IC50 = 0.08 μM) and achieved >50 % inhibition at 0.01 μM. Importantly, the inhibitory effects of these compounds on RANKL-induced osteoclast differentiation were not attributed to cytotoxicity, as evidenced by the minimal cytotoxicity of compound 18 at 10 μM. Mechanistic studies showed that compound 18 could dose-dependently suppress the expression of osteoclast marker genes (TRAP, CTSK) and proteins (c-Fos, MMP-9). Furthermore, in an ovariectomized (OVX) mouse model, compound 18 (10 and 20 mg/kg, intraperitoneally, i.p.) dose - dependently prevented bone loss by improving key micro-CT parameters and decreasing serum bone resorption markers (CTx). Overall, compound 18 emerged as a highly promising candidate for the treatment of RANKL-driven osteoporosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


