远端肽延伸由蛋白酶样连接酶和两个不同的载体蛋白
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
clothioamide (CTA)是一种有效的抗生素,具有独特的多硫酰胺支架,由反刍芽胞杆菌产生。与经典的非核糖体肽合成酶(NRPSs)不同,CTA的生物合成是通过非规范的独立酶进行的,后者使用模块化的腺苷化和缩合结构域。这个过程的核心是木瓜蛋白酶样连接酶CtaG,它催化两种不同的肽基载体蛋白(pcp)之间的酰胺键形成:CtaH,呈现对羟基苯甲酸(PHBA), CtaE,携带三β-丙氨酸((βAla)3)链。通过生化分析、化学探针、晶体学和突变分析,我们发现CtaG通过一种涉及酶结合中间体的乒乓机制起作用。单一底物隧道介导定向转移,使远端链延伸反映固相肽合成。基于结构的基因组挖掘揭示了在石蜡蛋白、丁松香和甲基黄烷素的生物合成途径中存在同源酶。总之,我们的发现揭示了以前被忽视的一类硫模板化连接酶,并为工程核糖体非依赖性肽装配线提供了机制蓝图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
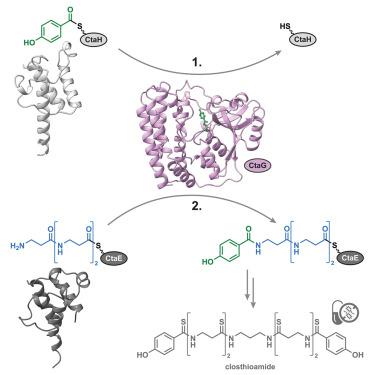
Distal peptide elongation by a protease-like ligase and two distinct carrier proteins
Closthioamide (CTA) is a potent antibiotic with a unique polythioamide scaffold produced by Ruminiclostridium cellulolyticum. Unlike classical non-ribosomal peptide synthetases (NRPSs), which use modular adenylation and condensation domains, CTA biosynthesis proceeds through non-canonical standalone enzymes. Central to this process is the papain-like ligase CtaG, which catalyzes amide bond formation between two distinct peptidyl carrier proteins (PCPs): CtaH, presenting para-hydroxybenzoic acid (PHBA), and CtaE, carrying a tri-β-alanine ((βAla)3) chain. Using biochemical assays, chemical probes, crystallography, and mutational analysis, we show that CtaG operates via a ping-pong mechanism involving an enzyme-bound intermediate. A single substrate tunnel mediates directional transfer, enabling distal chain elongation that mirrors solid-phase peptide synthesis. Structure-based genome mining revealed homologous enzymes in the biosynthetic pathways of petrobactin, butirosin, and methylolanthanin. Together, our findings uncover a previously overlooked class of thiotemplated ligases and provide a mechanistic blueprint for engineering ribosome-independent peptide assembly lines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


