通过Pt修饰触发电沉积CoNiFe LDH的固有催化活性,实现高效析氢反应
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
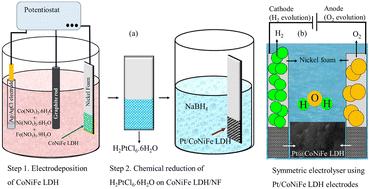
氢是一种清洁、可持续的能源载体,开发高效、耐用的析氢反应电催化剂是实现大规模制氢的关键挑战。在这项研究中,我们报告了两步合成铂修饰的CoNiFe层状双氢氧化物(Pt@CNF)电催化剂,旨在提高HER性能。场发射扫描电镜(FE-SEM)证实了铂纳米粒子在CoNiFe LDH (CNF)纳米片上的均匀分布。Pt的加入具有最佳的氢吸附能,在电流密度为10 mA cm−2时,将过电位从215 mV (CNF)显著降低到117 mV (Pt@CNF)。转换频率(TOF)从7.1 × 10−3 s−1大幅增加到14.6 × 10−3 s−1,进一步证明了Pt@CNF固有活性的增强。此外,与原始CNF相比,Pt@CNF具有更大的电化学表面积(ECSA)和更高的活性ECSA (AECSA),反映了更大的可达催化位点密度。Pt和CNF基质之间的协同作用提高了电荷转移动力学和催化耐久性,使Pt@CNF在100、200和400 mA cm - 2的高电流密度下分别提供298、370和441 mV的低过电位。这些发现突出了Pt@CNF作为下一代制氢技术的高性能HER电催化剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Triggering the intrinsic catalytic activity of electrodeposited CoNiFe LDH via Pt decoration for an efficient hydrogen evolution reaction
Hydrogen is widely recognized as a clean and sustainable energy carrier, and the development of efficient, durable electrocatalysts for the hydrogen evolution reaction (HER) remains a key challenge in realizing large-scale hydrogen production. In this study, we report a two-step synthesis of a platinum decorated CoNiFe layered double hydroxide (Pt@CNF) electrocatalyst, engineered to enhance HER performance. Field-emission scanning electron microscopy (FE-SEM) confirms the uniform distribution of Pt nanoparticles on CoNiFe LDH (CNF) nanosheets. The incorporation of Pt, possessing an optimal hydrogen adsorption energy, significantly reduces the overpotential from 215 mV (CNF) to 117 mV (Pt@CNF) at a current density of 10 mA cm−2. The enhanced intrinsic activity of Pt@CNF is further evidenced by a substantial increase in turnover frequency (TOF) from 7.1 × 10−3 s−1 to 14.6 × 10−3 s−1. Additionally, Pt@CNF exhibits a larger electrochemical surface area (ECSA) and higher active ECSA (AECSA) compared to pristine CNF, reflecting the greater density of accessible catalytic sites. The synergy between Pt and the CNF matrix improves both charge transfer kinetics and catalytic durability, enabling Pt@CNF to deliver low overpotentials of 298, 370, and 441 mV at high current densities of 100, 200, and 400 mA cm−2, respectively. These findings highlight the potential of Pt@CNF as a high-performance HER electrocatalyst for next-generation hydrogen production technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


