氧还原反应中o-B2N2单层负载双原子催化剂的双氮配位壳工程
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
单原子和双原子催化剂(SACs/ dac)已成为氧还原反应(ORR)的有希望的候选材料,但由于缺乏强大的底物来同时解决结构稳定性和活性问题,它仍然具有挑战性。本文利用密度泛函理论(DFT)计算,制备了一种具有窄带隙(0.66 eV)的新型二维正交二氮化硼(o-B2N2)单层,并对其作为ORR电催化剂的底物进行了评价。采用双过渡金属原子在双氮壳层(TM-N-N)内配位的协同策略来优化ORR性能。值得注意的是,所有基于三维过渡金属的TM2@o-B2N2单层膜都表现出优异的热稳定性和电化学稳定性。此外,在一系列TM2@o-B2N2单分子层中发现了双铁中心的o-B2N2 (Fe2@o-B2N2),具有优异的ORR性能。通过晶体轨道汉密尔顿居群(COHP)、差分电荷密度和分子轨道分析探测了电催化活性,揭示了性能增强的电子来源。Fe₂@o-B₂N₂的ORR活性增强源于Fe-N-N配位壳层中Fe-3d/N-2p轨道的强杂化。双氮层和双金属位点协同调节d波段中心,通过促进电子从反键态到键态的再分布,优化了关键物质(如*O₂/*OH)的吸附,优于传统的h-BN和n掺杂石墨烯。这项工作不仅确立了Fe2@o-B2N2作为一种优秀的ORR电催化剂,而且揭示了一种在电催化系统中具有广泛适用性的新型bn样衬底材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
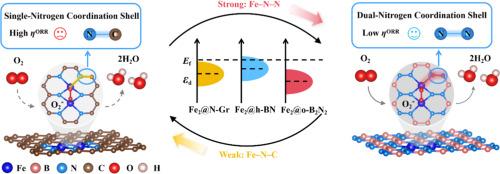
Dual-Nitrogen Coordination Shell Engineering in o-B2N2 Monolayer Supported Dual-Atom Catalysts for Oxygen Reduction Reaction
Single-atom and dual-atom catalysts (SACs/DACs) have emerged as promising candidates for the oxygen reduction reaction (ORR), but it is still challenging due to the absence of robust substrates for simultaneously addressing the structure stability and activity issues. Herein, by using density functional theory (DFT) computations, a novel two-dimensional orthorhombic diboron dinitride (o-B2N2) monolayer with a narrow bandgap (0.66 eV) was developed and evaluated as an electrocatalyst substrate for ORR. A synergistic strategy involving dual transition metal atoms coordinated within a dual-nitrogen shell (TM-N-N) was employed to optimize the ORR performance. Remarkably, all TM2@o-B2N2 monolayers based on 3d transition metals exhibit excellent thermal and electrochemical stability. Additionally, a di-iron centered o-B2N2 (Fe2@o-B2N2) was identified among a series of TM2@o-B2N2 monolayers, exhibiting superior ORR performance. The electrocatalytic activity was probed via crystal orbital Hamilton population (COHP), differential charge density, and molecular orbital analyses, revealing the electronic origins of the enhanced performance. The enhanced ORR activity of Fe₂@o-B₂N₂ originates from strong Fe-3d/N-2p orbital hybridization in the Fe-N-N coordination shell. The dual-nitrogen layer and di-metal sites synergistically modulate the d-band center, optimizing the adsorption of key species (e.g., *O₂/*OH) by facilitating electron redistribution from antibonding to bonding states, surpassing traditional h-BN and N-doped graphene counterparts. This work not only establishes Fe2@o-B2N2 as an excellent ORR electrocatalyst, but also unveils a novel BN-like substrate material with broad applicability in electrocatalytic systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


