由Col4a1突变引起的生物力学和成分基膜缺陷影响心脏形态和功能。
IF 4.8
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
IV型胶原是基底膜的主要成分,COL4A1和COL4A2基因的突变在临床上表现为一种可变的多系统疾病,称为COL4A1(古尔德)综合征。来自病例报告的证据支持该疾病的心脏成分,但影响心脏的表型和功能影响,其进展和潜在机制都仍然缺乏特征。事实上,基底膜(BM)在成人心脏病中的作用仍未得到充分探讨。我们着手解决这些知识空白,结合深入的表型和分子分析Col4a1突变对心脏生物学的小鼠模型(Col4a1+/svc)古尔德综合征。这揭示了形态学上的心脏缺陷,包括心肌细胞肥大,心肌和血管纤维化重构,心功能受损。Col4a1突变导致收缩和舒张功能障碍,并伴有左心室发达压降低。从机制上讲,我们发现这些缺陷是由于突变蛋白的分泌和BM缺陷,而不是胶原蛋白错误折叠和蛋白质毒性应激。基底膜缺陷导致前纤维化状态,纤维性胶原沉积增加,心脏僵硬,ECM成分缺陷。这些都伴随着涉及肌瘤形成、肌膜稳定性和心肌细胞代谢的通路调节的改变,建立了col4a1相关心脏病的分子特征。有趣的是,这一分子特征的各个方面,包括心脏代谢途径、心肌收缩调节和BM成分表达,与常见的心肌病如冠状动脉微栓塞、扩张型、缺血性和肥厚性梗阻性心肌病有共同之处。通过定义Gould综合征的分子和表型心脏成分,这些数据表明,基底膜对维持收缩和舒张功能至关重要,基底膜的改变导致纤维化反应。这些数据增加了对基底膜和IV型胶原蛋白在心脏生物学中的作用的认识,并突出了Gould综合征和常见成人心脏病之间的共同机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
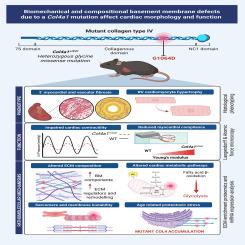
Biomechanical and compositional basement membrane defects due to a Col4a1 mutation affect cardiac morphology and function
Collagen IV is a major constituent of basement membranes and mutations in the genes COL4A1 and COL4A2 present clinically as a variable, multi-system disorder called COL4A1 (Gould) syndrome. Evidence from case reports supports a cardiac component to this disease, but the phenotypic and functional implications affecting the heart, their progression and underlying mechanisms all remain poorly characterised. Indeed, the role of the basement membrane (BM) in adult cardiac disease remains underexplored. We set out to address these knowledge gaps by combining in-depth phenotypic and molecular analyses of a Col4a1 mutation on cardiac biology in a murine model (Col4a1+/svc) of Gould Syndrome. This revealed morphological cardiac defects including cardiomyocyte hypertrophy with myocardial and vascular fibrotic remodelling that impaired cardiac function. The Col4a1 mutation causes systolic and diastolic dysfunction with reduced left ventricular developed pressure. Mechanistically, we show these defects are due to secretion of mutant protein and BM defects rather than collagen misfolding and proteotoxic stress. The BM defects lead to a pro-fibrotic state with increased fibrillar collagen deposition, cardiac stiffness, and ECM compositional defects. These are accompanied by altered regulation of pathways involved in sarcomere formation, sarcolemma stability and cardiomyocyte metabolism, establishing a molecular signature of COL4A1-related cardiac disease. Intriguingly, aspects of this molecular signature including cardiac metabolic pathways, regulation of cardiac muscle contraction and BM component expression, are shared with common cardiomyopathies such as coronary micro-embolism, and dilated, ischemic and hypertrophic obstructive cardiomyopathies. By defining the molecular and phenotypic cardiac components of Gould syndrome these data show that the BM is essential for maintaining systolic and diastolic function and that alterations to the BM leads to a fibrotic response. These data increase insight into the role of the basement membrane and collagen IV in cardiac biology, and highlights mechanisms shared between Gould syndrome and common adult cardiac disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Matrix Biology
生物-生化与分子生物学
CiteScore
11.40
自引率
4.30%
发文量
77
审稿时长
45 days
期刊介绍:
Matrix Biology (established in 1980 as Collagen and Related Research) is a cutting-edge journal that is devoted to publishing the latest results in matrix biology research. We welcome articles that reside at the nexus of understanding the cellular and molecular pathophysiology of the extracellular matrix. Matrix Biology focusses on solving elusive questions, opening new avenues of thought and discovery, and challenging longstanding biological paradigms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


