磁性功能化菲律宾天然沸石复合材料同时去除模拟废水中Cu2+、Ni2+和Zn2+的微观结构和吸附研究
IF 2.8
Q1 MATERIALS SCIENCE, CERAMICS
引用次数: 0
摘要
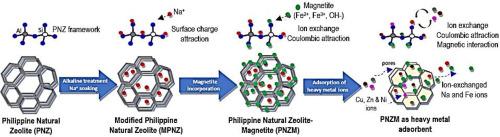
研究了表面改性菲律宾天然沸石(MPNZ)和磁功能化菲律宾天然沸石(PNZM)复合材料的微观结构特征和对重金属离子的吸附性能。SEM分析证实,PNZM表面存在分散的磁铁矿颗粒,使其表面更加粗糙和不规则,同时保留了其多孔结构。x射线衍射图显示磁铁矿尖晶石晶体结构和沸石骨架铝硅酸盐结构相对应的明显峰,表明复合材料具有良好的整体性。BET分析表明,加入磁铁矿后,MPNZ的比表面积由33.876 m2/g增加到45.052 m2/g。EDS表征证实了PNZM结构中Fe+离子的存在,增强了其阳离子交换能力(CEC)。MPNZ的Si/Al比值从3.75下降到3.37,表明MPNZ具有更多的负电荷,zeta电位结果表明MPNZ的表面电荷为(-)12.200 mV, PNZM的表面电荷为(-)20.854 mV。在单离子溶液中,PNZM对Ni2+、Cu2+和Zn2+的去除率分别为98.85%、99.99%和99.48%,均高于MPNZ。在混合离子溶液中,PNZM对Ni2+的去除率为91.17%,对Cu2+的去除率为97.90%,而Zn2+的去除率为97.98%,而MPNZ的去除率为99.99%。总的来说,加入磁铁矿使PNZM通过离子交换和库仑静电相互作用的协同机制去除重金属离子,从而功能化了PNZM可持续水处理的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Microstructure and adsorption studies on the simultaneous removal of Cu2+, Ni2+, and Zn2+ from simulated wastewater using magnetically functionalized Philippine natural zeolite composite
This study reports the microstructural characteristics and adsorption properties for heavy metal ions of the surface-modified Philippine natural zeolite (MPNZ) and magnetically functionalized Philippine natural zeolite (PNZM) composite. SEM analysis confirmed the presence of magnetite particles dispersed on PNZM, making its surface rougher and irregular while retaining its porous structure. X-ray diffractogram revealed distinct peaks corresponding to the spinel crystalline structure of magnetite and the aluminosilicate structure of the zeolite framework, suggesting a well-integrated composite material. BET analysis showed an increase in the surface area of MPNZ from 33.876 m2/g to 45.052 m2/g after adding magnetite. EDS characterization verified the strong presence of Fe+ ions in the PNZM structure, enhancing its cation-exchange capacity (CEC). The Si/Al ratio of MPNZ decreased from 3.75 to 3.37, indicating a more negative charge, supported by zeta potential results that showed surface charges of (-)12.200 for MPNZ to (-) 20.854 mV for PNZM. In single ion solutions, PNZM obtained a removal uptake of 98.85 %, 99.99 % and 99.48 % for Ni2+, Cu2+and Zn2+ respectively, which are higher than MPNZ. In mixed-ion solutions, PNZM also showed improved adsorption with removal rates of 91.17 % for Ni2+ and 97.90 % for Cu2+, although Zn2+ uptake decreased to 97.98 % compared to the 99.99 % of MPNZ. Overall, incorporating magnetite has functionalized the ability of PNZM for sustainable water treatment by removing heavy metal ions through the synergistic mechanism of ion-exchange and Coulombic electrostatic interactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Open Ceramics
Materials Science-Materials Chemistry
CiteScore
4.20
自引率
0.00%
发文量
102
审稿时长
67 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


