合成条件对二水合草酸镍结构无序性和对称性的影响
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
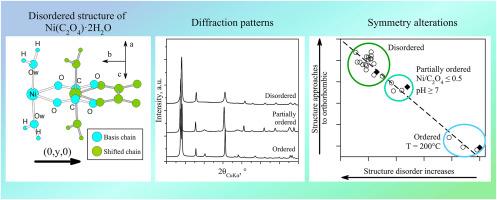
二水合草酸镍α-NiC2O4∙2H2O(空间群C2/c)属于通式为M2+(C2O4)∙2H2O (M = Fe, Mn, Mg, Zn, Ni, Co)的草酸盐族,被称为洪堡丁族矿物。为了阐明结构紊乱的模式,我们在广泛的条件下(pH = 1.5-9.5, Ni/C2O4 = 0.17-4, T = 25-200°C)从水溶液中合成了草酸镍二水合物,并使用粉末x射线衍射(Rietveld细化)和扫描电子显微镜(edx光谱)对合成产物进行了探索。结果表明,NiC2O4∙2H2O的结构紊乱会影响衍射图样和晶胞参数。我们发现了一系列单斜结构,它们的无序程度各不相同。过量的草酸离子、弱碱性的pH值以及结晶介质的温度对单斜型草酸镍结构的有序性有积极的影响。结构失序与其偏离正交对称之间存在反比关系。本文提出了Ni(C2O4)∙2H2O草酸盐中空间群Cccm、Fddd和C2/c之间跃迁的结构机制(多态性、不对称)。该研究有助于我们对洪堡丁基草酸盐晶体化学的认识,并可用于未来的生物技术。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural disorder and symmetry of nickel oxalate dihydrate affected by synthesis conditions
Nickel oxalate dihydrate α-NiC2O4∙2H2O (space group C2/c) belongs to a family of oxalates with general formula M2+(C2O4)∙2H2O (M = Fe, Mn, Mg, Zn, Ni, Co), known as humboldtine group minerals. To elucidate patterns of structural disorder we have synthesized Ni-oxalate dihydrate from water solution in a wide range of conditions (pH = 1.5–9.5, Ni/C2O4 = 0.17–4, T = 25–200 °C) and explored the synthesis products using powder X-ray diffraction including Rietveld refinement and scanning electron microscopy with EDX-spectroscopy. It was revealed that structural disorder of NiC2O4∙2H2O affects the diffraction patterns and unit cell parameters. We have found an entire series of monoclinic structures, different by their degree of disorder. Excess of oxalate ions, weak-alkaline pH and especially temperature of crystallization medium are positively affecting the ordering of monoclinic nickel oxalate structure. There is an inverse correlation between structure disorder and its deviation from orthorhombic symmetry. Structural mechanisms of transitions between space groups Cccm, Fddd and C2/c in Ni(C2O4)∙2H2O oxalates (polymorphism, desymmetrization) have been suggested. The study contributes to our understanding of crystal chemistry of humboldtine group oxalates and could be used in biotechnologies in future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


