富含乳酸杆菌的宫颈阴道微生物组与女性较低的BV、HPV和细胞学结果相关
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
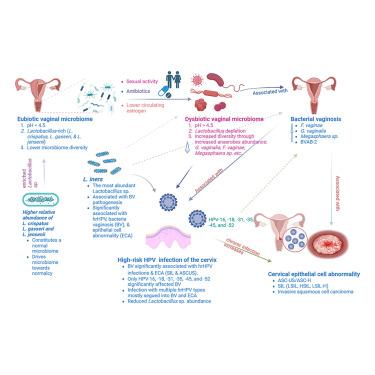
宫颈阴道微生物组调节对细菌性阴道病(BV)、高危人乳头瘤病毒(hrHPV)感染和宫颈癌前上皮细胞异常的易感性。我们回顾性分析了15 607例美国妇女(14-95岁;32个州)的qpcr检测的宫颈阴道标本,并整合了微生物组丰度、hrHPV基因分型、pap细胞学和人口统计学。BV在53%的样本中存在,hrHPV在11%的样本中存在。在bv阴性和细胞学正常(NILM)的样本中富集了crispatus、L. gasseri和L. jensenii,而L. iners和bv相关的厌氧菌与hrHPV和细胞学异常共存。机器学习模型证实,年龄、hrHPV状态和crispatus丰度是BV和细胞学结果的最强多变量预测因子(BV AUROC≈0.97)。相互作用分析显示,特定hrHPV基因型与加德纳菌/范尼赫西之间存在协同关联,这进一步增加了细胞学风险。这些发现强调了微生物组分析的临床价值,并支持益生菌策略,促进保护性乳酸杆菌群落减少BV和hrhpv相关的宫颈病理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lactobacillus-rich cervicovaginal microbiome associated with lower BV, HPV, and cytology outcomes in women
The cervicovaginal microbiome modulates susceptibility to bacterial vaginosis (BV), high-risk human papillomavirus (hrHPV) infection, and epithelial cell abnormalities that precede cervical cancer. We retrospectively analyzed 15 607 qPCR-profiled cervicovaginal specimens from U.S. women (ages 14–95; 32 states) and integrated microbiome abundances, hrHPV genotyping, Pap-cytology, and demographics. BV was present in 53% and hrHPV in 11% of samples. Lactobacillus crispatus, L. gasseri, and L. jensenii were enriched in BV-negative and cytologically normal (NILM) samples, whereas L. iners and BV-associated anaerobes co-occurred with hrHPV and abnormal cytology. Machine-learning models confirmed age, hrHPV status, and L. crispatus abundance as the strongest multivariate predictors of BV and cytological outcomes (BV AUROC ≈0.97). Interaction analyses revealed synergistic associations between specific hrHPV genotypes and Gardnerella/Fannyhessea that further increased cytological risk. These findings underscore the clinical value of microbiome profiling and support probiotic strategies that promote protective Lactobacillus communities to reduce BV and hrHPV-related cervical pathology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


