一种新型手性配体:设计及其催化不对称反应性能评价
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以8-羟基-2-甲基喹啉为起始原料,l-脯氨酸衍生,设计并制备了一种新型脯氨酸手性配体。这两部分的偶联产生了理想的手性配体,分离收率高。以二乙基锌对芳香醛的对映选择性加成反应为模型反应,评价了这些手性配体的性能。在优化条件下,二乙基锌对芳香醛进行了对映选择性加成,得到了高收率的手性醇,收率高达97%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
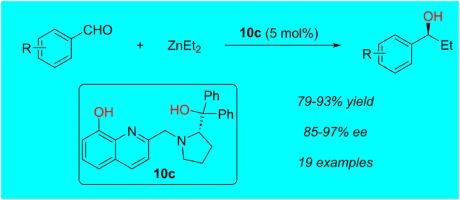
A new type of chiral ligands: Design and the evaluation of their performance in catalytic asymmetric reactions
A new type of proline-based chiral ligands were designed and prepared starting from 8-hydroxy-2-methylquinoline and derivatization of l-proline. Coupling of these two parts yielded the desired chiral ligands in good isolated yields. The performance of these chiral ligands was evaluated using enantioselective addition of diethylzinc to aromatic aldehydes as model reactions. Under the optimized conditions, enantioselective addition of diethylzinc to aromatic aldehydes proceeded readily, giving the desired chiral alcohols in high yields and up to 97 % ee's.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


