硼掺杂多孔生物炭活化过硫酸盐高效去除磺胺甲恶唑:同时增强吸附和非自由基途径
IF 6.7
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
催化剂的性质和活化途径是过硫酸盐基深度氧化工艺的关键。本研究成功合成了具有吸附-催化双重功能的硼掺杂多孔生物炭(BPC-0.25),以激活过硫酸氢盐(PDS)降解磺胺甲恶唑(SMX)。结果表明,BPC-0.25通过吸附在30 min内去除83%的SMX,并通过与PDS氧化的协同作用在20 min内完全降解。同时,BPC-0.25/PDS系统具有良好的抗干扰能力,能够适应复杂的水质条件。猝灭实验、EPR测试和电化学分析表明,BPC-0.25对PDS的活化以单重态氧(1O2)的生成为主,以电子转移过程(ETP)为补充。硼的掺入显著提高了催化剂的吸附能力和传质能力,这是由于介孔结构的形成和表面亲和力的提高。同时,羰基(C=O)和硼掺杂物的含量增加,两者都具有较高的反应活性,从而显著提高了催化性能和ETP效率。结果进一步确定了CO、结构缺陷和BC3是BPC-0.25/PDS体系的主要活性位点。此外,通过质谱分析和理论计算提出了SMX可能的降解途径,并用ECOSAR预测了中间体的生态毒性。总体而言,本研究为设计高效环保的无金属催化剂提供了创新策略,为PDS的活化机理提供了新的见解,并为废水修复提供了有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
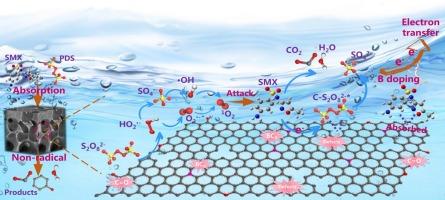
Efficient removal of sulfamethoxazole by boron-doped porous biochar-activated persulfate: simultaneously enhanced adsorption and non-radical pathways
The properties of catalysts and activation pathway are crucial in persulfate-based advanced oxidation processes. In this study, boron-doped porous biochar (BPC-0.25), featuring adsorption-catalysis dual function, was successfully synthesized to activate peroxydisulfate (PDS) for sulfamethoxazole (SMX) degradation. The results showed that BPC-0.25 removed 83 % of SMX in 30 min via absorption and achieved complete degradation in 20 min through the synergistic effect with PDS oxidation. Meanwhile, the BPC-0.25/PDS system exhibited good anti-interference ability to adapt to complex water quality conditions. The quenching experiments, EPR tests, and electrochemical analyses collectively revealed that the PDS activation by BPC-0.25 was dominated by the generation of singlet oxygen (1O2) and supplemented by electron transfer process (ETP). Remarkably, boron doping enhanced the adsorption capacity and mass transfer of the catalysts due to the formation of mesoporous structure and improvement of surface affinity. Simultaneously, the content of carbonyl group (C=O) and boron doped species were increased, both of which possessed high reactivity, thereby significantly improving the catalytic performance and ETP efficiency. The results further identified that C![]() O, structural defects, and BC3 were the main active sites in the BPC-0.25/PDS system. Besides, possible degradation pathways of SMX were proposed by mass spectrometry and theoretical calculations, while the ecotoxicity of the intermediates was predicted by ECOSAR. Overall, this work offers innovative strategies for designing efficient and environmentally friendly metal-free catalysts, provides novel insights into the activation mechanism of PDS, and presents effective approaches for wastewater remediation.
O, structural defects, and BC3 were the main active sites in the BPC-0.25/PDS system. Besides, possible degradation pathways of SMX were proposed by mass spectrometry and theoretical calculations, while the ecotoxicity of the intermediates was predicted by ECOSAR. Overall, this work offers innovative strategies for designing efficient and environmentally friendly metal-free catalysts, provides novel insights into the activation mechanism of PDS, and presents effective approaches for wastewater remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


