塞来昔布负载间充质干细胞衍生的外泌体改善激光诱导的兔模型脉络膜新生血管
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-11
DOI:10.1016/j.jddst.2025.107514
引用次数: 0
摘要
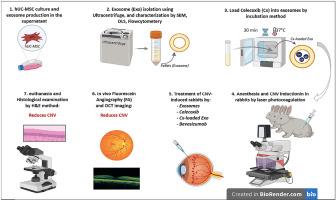
新生血管性年龄相关性黄斑变性(nAMD)是中央视力失明的原因,与脉络膜新生血管(CNV)有关。本研究的目的是评估载塞来昔布外泌体的治疗能力,并在激光诱导的兔CNV模型中直接比较它们与其他三种方法(游离塞来昔布、纯外泌体和贝伐单抗)的疗效。采用超离心分离方法分离人脐带间充质干细胞(hUC-MSCs)外泌体,并采用动态光散射、场发射扫描电镜和流式细胞术对其进行鉴定。通过被动孵育将塞来昔布装载到外泌体中。将18只家兔分为6组:健康对照组、未治疗组和4个治疗组。热激光光凝诱导CNV。通过荧光素血管造影(FA)、光学相干断层扫描(OCT)和组织病理学分析检查治疗效果。外泌体显示出成功的结构和表面标记谱,塞来昔布的有效负载效率为18.08±0.21%。与未治疗对照组相比,所有治疗组CNV面积和血管渗漏均显著减少(p < 0.0001)。接受塞来昔布加载外泌体的组在成像结果上表现出最显著的改善,在OCT和FA分析中分别减少了60.43%的CNV厚度和75.95%的荧光素泄漏。组织病理学评估进一步证实了治疗效果,显示CNV厚度减少了72.79%。通过外泌体给药塞来昔布显著提高了其治疗CNV的效果。这种联合策略利用塞来昔布的抗炎和抗血管生成能力以及外泌体的再生能力和靶向递送优势,为未来治疗nAMD提供了一个有希望的方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Celecoxib-loaded mesenchymal stem cell-derived exosomes ameliorate laser-induced choroidal neovascularization in a rabbit model
Neovascular age-related macular degeneration (nAMD), cause of central vision blindness, is associated with choroidal neovascularization (CNV). The objective of this research was to assess the treatment capabilities of Celecoxib-loaded exosomes, and directly compare their efficacy with three other approaches, free Celecoxib, pure exosomes, and Bevacizumab, in a laser-induced CNV rabbit model. Human umbilical cord mesenchymal stem cells (hUC-MSCs) derived exosomes were isolated by ultracentrifugation and identified means of dynamic light scattering, field emission scanning electron microscopy, and flow cytometry. Celecoxib was loaded into exosomes through passive incubation. Eighteen rabbits were divided into six groups: a healthy control group, an untreated CNV group, and four treatment groups. CNV was induced by thermal laser photocoagulation. Therapeutic effects were examined via fluorescein angiography (FA), optical coherence tomography (OCT), and histopathological analysis. The exosomes showed successful structural and surface marker profiles, and Celecoxib was effectively loaded with an efficiency of 18.08 ± 0.21 %. All treatment groups exhibited significant reductions (p < 0.0001) in CNV area and vascular leakage relative to the untreated control. The group receiving Celecoxib-loaded exosome showed the most substantial improvement in imaging outcomes, reducing CNV thickness by 60.43 % and fluorescein leakage by 75.95 % in OCT and FA analyses, respectively. Histopathological evaluation further confirmed therapeutic efficacy, showing a 72.79 % reduction in CNV thickness. Delivering Celecoxib via exosomes markedly enhanced its therapeutic performance in CNV. This combined strategy leverages the anti-inflammatory and anti-angiogenic capabilities of Celecoxib with the regenerative capacity and targeted delivery advantages of exosomes, offering a promising direction for future treatments of nAMD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


