超越凋亡:铜/铁配体纳米复合物通过铜下沉和铁下沉引导癌症治疗
IF 23.5
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
利用不同的金属依赖性细胞死亡途径为克服癌症细胞凋亡抵抗提供了一种有希望的策略。本综述探讨了金属配体纳米复合物的最新进展,这是一种复杂的平台,旨在克服全身毒性和自由金属离子的生物利用度差,选择性地触发两种不同的细胞死亡途径:铜中毒(铜(Cu)依赖的蛋白质聚集)和铁中毒(铁(Fe)催化的脂质过氧化)。本综述的中心论点是配体配位化学在调整物理化学性质方面的关键作用,包括稳定性、氧化还原电位和控制这些纳米配合物机制特异性的金属释放动力学。本文根据这些纳米配合物的配位供体原子(O, N, S)对其进行了系统的分类,以说明分子结构如何转化为选择性抗癌功效。双模Cu/Fe纳米复合物的发展进一步说明了通过两种途径的协同激活来发挥放大的治疗效果。因此,本综述为新一代金属基纳米治疗药物的合理设计提供了一个战略框架,为精准肿瘤学开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
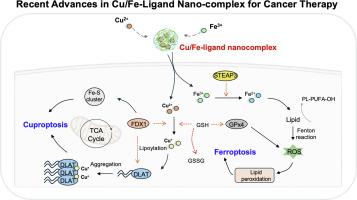
Beyond apoptosis: Navigating Cancer therapy with cu/Fe-ligand Nano-complexes through Cuproptosis and Ferroptosis
Exploiting distinct metal-dependent cell death pathways offers a promising strategy to overcome apoptosis resistance in cancer. This review explores recent advances in metal-ligand nano-complexes, sophisticated platforms engineered to overcome the systemic toxicity and poor bioavailability of free metal ions, selectively triggering two distinct cell death pathways: cuproptosis (copper (Cu)-dependent protein aggregation) and ferroptosis (iron (Fe)-catalyzed lipid peroxidation). The central thesis of this review is the pivotal role of ligand coordination chemistry in tailoring the physicochemical properties, including stability, redox potential, and metal release kinetics that control the mechanistic specificity of these nano-complexes. This review provides a systematic classification of these nano-complexes based on their coordinating donor atoms (O, N, S) to illustrate how molecular configuration translates into selective anticancer efficacy. The development of dual-mode Cu/Fe nano-complexes is further illustrated to exert amplified therapeutic efficacy through the synergistic co-activation of both pathways. Therefore, this review provides a strategic framework for the rational design of next-generation metal-based nanotherapeutics, opening new avenues for precision oncology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


