结合内辐射和时空释放IL-12的双层纳米纤维涂层支架促进抗肿瘤免疫反应
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
内照射(IR)是以碘-125 (125I)粒子为辐射源,是临床上确立的靶向治疗恶性肿瘤的一种方式。除了在肿瘤细胞中诱导DNA损伤外,红外治疗还可以通过免疫原性细胞死亡(ICD)引发全身抗肿瘤免疫反应,即所谓的体外效应。然而,放射性输出的逐渐下降和IR治疗固有的有限免疫原性损害了其整体疗效,在临床环境中很少观察到体外效应。为了克服这些挑战,我们开发了一种双层纳米纤维涂层支架,将基于125I种子的近距离治疗与持续的、时空控制的白介素-12 (IL-12)递送结合起来。利用fda批准的PLGA和PEO聚合物,内层被设计用于装载IL-12,而外层作为调节屏障,实现双相释放,持续递送细胞因子长达14 天。体内研究表明,IL-12@IRS(负载il -12的125I内放射支架)有效地增强了抗原呈递,引发了肿瘤原位接种效应,从而促进了持久的局部和全身抗肿瘤免疫。这种免疫放射治疗平台不仅改善了局部肿瘤控制,而且为克服免疫抵抗和诱导强大的全身抗肿瘤作用提供了一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
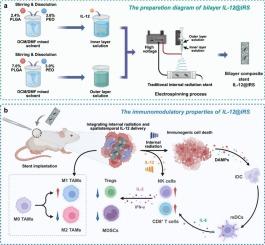
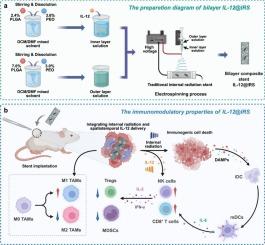
Bilayer nanofiber-coated stent integrating internal radiation and spatiotemporal IL-12 release promotes antitumor immune response
Internal radiation (IR), utilizing iodine-125 (125I) seeds as a radiation source, is a clinically established modality for targeted treatment of malignant tumors. In addition to inducing DNA damage in tumor cells, IR therapy can trigger systemic antitumor immune responses, known as the abscopal effect, through immunogenic cell death (ICD). However, the gradual decline in radioactive output and the inherently limited immunogenicity of IR therapy compromise its overall efficacy, with the abscopal effect rarely observed in clinical settings. To overcome these challenges, we developed a bilayer nanofiber-coated stent integrating 125I seed-based brachytherapy with sustained, spatiotemporally controlled delivery of interleukin-12 (IL-12). Utilizing FDA-approved PLGA and PEO polymers, the inner layer was engineered for IL-12 loading, while the outer layer acted as a regulatory barrier, achieving a biphasic release profile with sustained cytokine delivery for up to 14 days. In vivo studies demonstrated that the IL-12@IRS (IL-12-loaded 125I internal radiation stent) effectively enhanced antigen presentation and elicited a tumor in situ vaccination effect, thereby promoting durable local and systemic antitumor immunity. This immuno-radiotherapeutic platform not only improves local tumor control but also offers a promising strategy for overcoming immune resistance and inducing robust systemic antitumor effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


