Lys49 pla2样毒素的分子动力学:对溶液和膜结合构象的见解。
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
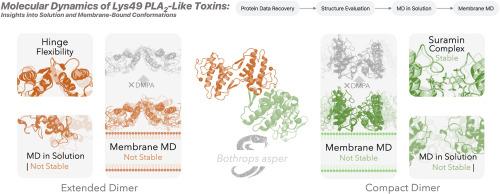
蛇咬伤是一种被忽视的热带疾病,可导致死亡和残疾。Lysine 49 phospholipase A2-like (Lys49 PLA2-like)蛋白是多种毒蛇毒液中丰富的毒素,在毒蛇毒性中起重要作用。它们通过一种独立于磷脂水解的机制,通过破坏质膜诱导骨骼肌坏死。分析了Bothrops aspper Lys49 pla2样蛋白的序列、折叠和四级结构,并与PDB中其他毒蛇Lys49 PLA2s的二聚体几何形状进行了比较。序列和褶皱主要保守,而第四纪结构则不保守。除了一个例外,所有的第四系结构都可以分为两类:紧凑型和扩展型。用分子动力学模拟了B. aspper扩展和紧凑的Lys49 PLA2构象在溶液和膜双分子层中,并根据力学建议和实验数据进行了仔细研究。两种构象在溶液中解离成单体。带负电荷的磷脂含量越高,膜结合越稳定,这是通过包含DMPA实现的。扩展二聚体在纯POPC中解吸,吸附在POPC/DMPA(9:1)时保持二聚体构象,在POPC/DMPA(1:1)中解离成单体。然而,在后一种情况下,蛋白质仍然被吸附。紧凑二聚体在纯POPC双分子层中从膜上解吸,在POPC/DMPA(1:1)中失去构象,解离成POPC/DMPA(9:1)组成的单体。单体在垂直方向(致密状)和水平方向(延伸状)之间振荡。这些情景表明,目前关于Lys49 pla2样蛋白破坏膜机制的假设与证据并不完全一致,需要考虑其他可能性。我们的观察结果表明,Lys49 PLA2s可以作为单体,但也可能在膜相互作用中结合成高阶低聚物。这种高度复杂的分子场景与最近的证据一致,表明膜促进蛋白质寡聚化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular dynamics of Lys49 PLA2-like toxins: Insights into solution and membrane-bound conformations
Snakebite envenoming is a neglected tropical disease that causes death and disability. Lysine 49 phospholipase A2-like (Lys49 PLA2-like) proteins are abundant toxins in many viperid venoms and play a significant role in their toxicity. They induce skeletal muscle necrosis by disrupting the plasma membrane through a mechanism independent of phospholipid hydrolysis. X-ray structures of Bothrops asper Lys49 PLA2-like proteins were analysed for sequence, folding and quaternary structure, and compared with dimer geometries of Lys49 PLA2s from other viper species in the PDB. Sequence and folding were mainly conserved, whilst quaternary structure was not. With a single exception, all quaternary structures fell into two categories: compact and extended.
The B. asper extended and compact Lys49 PLA2 conformations were simulated in solution and in a membrane bilayer with molecular dynamics and scrutinised in light of mechanistic proposals and experimental data. Both conformations dissociated into monomers in solution. Membrane binding was more stable with higher fractions of negatively charged phospholipids, achieved through the inclusion of DMPA. The extended dimer desorbed in pure POPC, kept its dimeric conformation when adsorbed to POPC/DMPA (9:1), and dissociated into monomers in POPC/DMPA (1:1). Still, the protein remained adsorbed in the latter case. The compact dimer desorbed from the membrane in the pure POPC bilayer, lost its conformation in POPC/DMPA (1:1), and dissociated into monomers in a POPC/DMPA (9:1) composition. The monomers oscillated between vertical (compact-like) and horizontal (extended-like) orientations.
These scenarios show that the current hypotheses for the membrane disruption mechanism by Lys49 PLA2-like proteins are not entirely aligned with the evidence, and alternative possibilities need to be considered. Our observations suggest that Lys49 PLA2s could act as monomers but may also associate into higher-order oligomers during membrane interaction. This highly complex molecular scenario aligns with recent evidence indicating that the membrane promotes protein oligomerisation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


