负载voc的fau型Y型沸石的热解吸动力学和骨架演化:原位XRPD研究
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本文报道了在动态(298 ~ 973 K)和等温(443 ~ 523 K)条件下,用原位同步x射线粉末衍射研究了高硅fu型Y型沸石(SiO2/Al2O3≈200)对甲苯和氯苯的解吸动力学。动态XRPD数据显示了一个渐进的单位细胞收缩(~ 0.4 - 0.5%)和(111)反射强度的增加,与挥发性有机化合物的逐渐释放和孔隙疏散相一致。使用Avrami-Erofeev和Arcenegui-Troya动力学模型计算活化能,后者包含归因于合作效应的非arrhenius行为。与甲苯(17.31±0.66 kJ/mol)相比,氯苯的活化能(28.13±3.93 kJ/mol)更高,这是由于四极-阳离子相互作用更强,旋转熵减少,以及分子拥挤引起的协同解吸障碍。这些发现为VOC解吸过程中主客体相互作用和框架稳定性提供了基本的结构见解,为环境应用的可再生沸石吸附剂的设计提供了信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
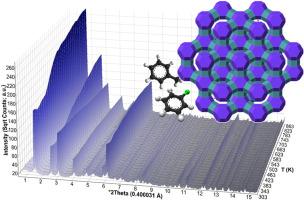
Thermal desorption kinetics and framework evolution in VOC-loaded FAU-Type zeolite Y: An in situ XRPD study
This study reports a detailed investigation of the desorption kinetics of toluene and chlorobenzene from a high-silica FAU-type Y zeolite (SiO2/Al2O3 ≈ 200) by in situ synchrotron X-ray powder diffraction under dynamic (298–973 K) and isothermal (443–523 K) conditions. Dynamic XRPD data reveal a progressive unit cell contraction (∼0.4–0.5 %) and an increase in the intensity of the (111) reflection, consistent with the gradual release of VOCs and pore evacuation. Activation energies were calculated using Avrami–Erofeev and Arcenegui–Troya kinetic models, the latter incorporating non-Arrhenius behavior attributed to cooperative effects. The higher activation energy observed for chlorobenzene (28.13 ± 3.93 kJ/mol) compared to toluene (17.31 ± 0.66 kJ/mol) is attributed to stronger quadrupole–cation interactions, reduced rotational entropy, and cooperative desorption barriers arising from molecular crowding. These findings provide fundamental structural insights into host–guest interactions and framework stability during VOC desorption, informing the design of regenerable zeolite adsorbents for environmental applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


