揭示小儿炎症性肠病中硫嘌呤诱导的胰腺炎的细胞机制:来自诱导多能干细胞模型的见解
IF 7.5
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
硫嘌呤是治疗炎症性肠病的有效药物,但其使用受到副作用的限制,如胰腺炎,其机制尚不清楚,在儿童中可能更严重。本研究利用来自儿童炎症性肠病患者的诱导多能干细胞,以个性化的方式研究了硫嘌呤诱导的胰腺炎机制。纳入10名儿童患者,5名发展为胰腺炎(病例),5名未发展为胰腺炎(对照组)。使用患者特异性干细胞及其胰腺分化对应体评估硫嘌呤的细胞毒性,通过液相色谱-串联质谱法量化代谢物水平,并通过western-blot法评估硫嘌呤的药效学。统计分析采用学生t检验或双向方差分析,然后采用Bonferroni事后检验进行多重比较。细胞毒性试验显示,病例的干细胞和胰腺细胞中有较高的硫鸟嘌呤细胞毒性;病例的胰腺细胞对巯基嘌呤也更敏感。此外,干细胞上的硫鸟嘌呤处理产生了单磷酸硫鸟嘌呤及其甲基化形式,但它们的浓度在两组之间没有显著差异。此外,TPMT基因在干细胞中表达较高,但在胰腺细胞中未见差异。HPRT、NUDT15、ITPA、PACSIN2的表达差异无统计学意义。最后,Rac1蛋白浓度在病例干细胞和对照组中相似,但病例胰腺细胞的Rac1表达明显更高。这些发现表明,硫嘌呤细胞毒性差异可能与干细胞的药代动力学有关,而胰腺细胞中Rac1表达的改变可能导致胰腺炎,这暗示了干细胞和分化细胞之间的不同机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
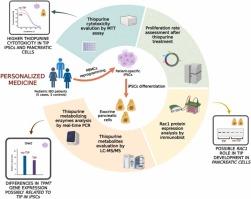
Unraveling the cellular mechanisms of thiopurine-induced pancreatitis in pediatric inflammatory bowel disease: Insights from induced pluripotent stem cell models
Thiopurines are effective drugs for inflammatory bowel disease, but their use is limited by side effects such as pancreatitis, whose mechanism remains unknown and may be more severe in children. This study investigated in a personalized way thiopurine-induced pancreatitis mechanism using induced pluripotent stem cells from pediatric inflammatory bowel disease patients.
Ten pediatric patients, five developing pancreatitis (cases) and five without it (controls), were enrolled. Patient-specific stem cells and their pancreatic differentiated counterparts were used to evaluate thiopurine cytotoxicity, to quantify metabolites levels by liquid chromatography-tandem mass spectrometry, and to assess thiopurine pharmacodynamics by western-blot assay. Statistical analyses were performed applying Student’s t-test or two-way ANOVA followed by Bonferroni’s post-hoc test for multiple comparisons. Cytotoxicity assays revealed higher thioguanine cytotoxicity in stem and pancreatic cells from cases; pancreatic cells from cases were also more sensitive to mercaptopurine. Moreover, thioguanine treatment on stem cells produced thioguanosine monophosphate and its methylated form, but their concentration did not differ significantly between the groups. In addition, higher TPMT gene expression was observed in stem cells from cases, but no differences were observed in pancreatic cells. No significant differences were detected in HPRT, NUDT15, ITPA, or PACSIN2 expression. Lastly, Rac1 protein concentration was similar in stem cells from cases and controls, but pancreatic cells from cases exhibited significantly higher Rac1 expression. These findings suggest that thiopurine cytotoxicity differences might be linked to pharmacokinetics in stem cells, while altered Rac1 expression in pancreatic cells might contribute to pancreatitis, implicating distinct mechanisms between stem and differentiated cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.90
自引率
2.70%
发文量
1621
审稿时长
48 days
期刊介绍:
Biomedicine & Pharmacotherapy stands as a multidisciplinary journal, presenting a spectrum of original research reports, reviews, and communications in the realms of clinical and basic medicine, as well as pharmacology. The journal spans various fields, including Cancer, Nutriceutics, Neurodegenerative, Cardiac, and Infectious Diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


