具有增强可见光催化性能的磁性锰铁钒氧化物/改性沸石纳米复合材料的一锅水热合成与表征
IF 4.9
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究通过一锅水热法成功合成了一种新型磁性杂化纳米材料(MnFe2O4/FeVO4/十六烷基三甲基溴化铵(CTAB)表面活性剂改性沸石),该材料在降解苯并噻吩(BT)和光催化性能方面表现出优异的性能。为了优化纳米复合材料的结构,提高其氧化脱硫性能,合成了几种不同纳米结构与改性沸石比例的样品,包括样品1:1 (S1:1)、样品2:1 (S1:1)、样品4:1 (S4:1)和样品8:1 (S8:1),并通过XRD、EDS、FESEM、TEM、BET、DRS和VSM分析对其进行了表征。合成的纳米复合材料具有优异的光催化活性,在可见光照射下具有较高的除硫效率。在污染物降解方面,对不同样品的除硫效率进行了评价。结果表明:MnFe2O4(65%)、S1:1 (Fe0.11V2O5.16/Mn2V2O7/改性沸石(1:1))(85%)、S2:1 (MnFe2O4/Mn2V2O7/改性沸石(2:1))(90%)、S4:1 (MnFe2O4/Fe0.5V3.5O8/改性沸石(4:1))(98%)、S8:1 (MnFe2O4/FeVO4/改性沸石(8:1))(100%)。S8:1 (MnFe2O4/FeVO4/改性沸石(8:1))对柴油中硫的去除率达到100%。MnFe2O4和FeVO4之间的协同作用显著改善了污染物的吸附和光催化降解。利用合成的纳米复合材料对含硫化合物在可见光催化下的降解行为进行动力学研究。实验数据采用了零阶、一阶和二阶三种动力学模型。其中,S8:1的一阶模型相关性最高(R2 = 0.9939),表明其存在表面控制的化学吸附机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
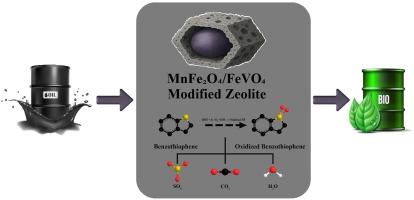
One-pot hydrothermal synthesis and characterization of magnetic Mn–Fe–V oxide/modified zeolite nanocomposite with enhanced visible-light photocatalytic properties
In this study, a novel type of magnetic hybrid nanomaterial (MnFe2O4/FeVO4/modified zeolite by Cetyl trimethyl ammonium bromide (CTAB) surfactant) was successfully synthesized via a one-pot hydrothermal method to meet this goal, demonstrating excellent performance in the degradation of benzothiophene (BT) and increasing photocatalytic properties. To optimize the structure of the nanocomposite and enhance its performance in oxidative desulfurization, several samples with different ratios of nanostructure to modified zeolite, including sample 1:1 (S1:1), sample 2:1 (S2:1), sample 4:1 (S4:1), and sample 8:1 (S8:1) were synthesized and characterized by XRD, EDS, FESEM, TEM, BET, DRS and VSM analyses. The synthesized nanocomposite exhibited superior photocatalytic activity and achieved high sulfur removal efficiency under visible light irradiation. In terms of pollutant degradation, the sulfur removal efficiency across various samples was evaluated. The results yielded the following percentages: MnFe2O4 (65 %), S1:1 (Fe0.11V2O5.16/Mn2V2O7/modified zeolite (1:1)) (85 %), S2:1 (MnFe2O4/Mn2V2O7/modified zeolite (2:1)) (90 %), S4:1 (MnFe2O4/Fe0.5V3.5O8/modified zeolite (4:1)) (98 %), and S8:1 (MnFe2O4/FeVO4/modified zeolite (8:1)) (100 %). S8:1 (MnFe2O4/FeVO4/modified zeolite (8:1)) was also found to remove 100 % of sulfur from diesel fuel. The synergistic interaction between MnFe2O4 and FeVO4 significantly improved pollutant adsorption and photocatalytic degradation. Kinetic investigations were conducted to evaluate the degradation behavior of sulfur-containing compounds under visible-light photocatalysis using the synthesized nanocomposites. Three kinetic models including zero-order, first-order and second-order were applied to the experimental data. Among them, the first order model exhibited the highest correlation (R2 = 0.9939) for S8:1, indicating a surface-controlled chemisorption mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


