线粒体功能障碍驱动zbp1介导的PANoptosis增加心力衰竭伴保留射血分数相关心房颤动的易感性
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
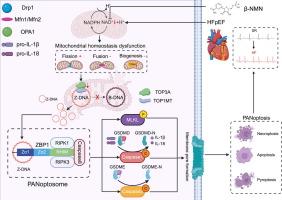
心力衰竭伴保留射血分数(HFpEF)常并发心房颤动(AF),但其潜在的分子机制仍不明确。线粒体功能障碍驱动zbp1介导的PANoptosis对于了解hfpef相关AF的进展和探索新的治疗途径至关重要。目的通过检测线粒体Z-DNA (mtZ-DNA),探讨Z-DNA结合蛋白1 (ZBP1)在HFpEF相关性af中作为连接线粒体功能障碍与PANoptosis的关键介质的作用。方法采用多种体内和体外实验方法,主要包括HFpEF小鼠模型建立、组织学染色、RNA测序、Western blotting、共免疫沉淀、透射电镜(TEM)和共聚焦成像。结果在“双击”HFpEF小鼠模型中,我们观察到AF易感性随着建模时间的延长而增加。此外,生物信息学分析和体内和体外研究强调了进行性zbp1介导的PANoptosis伴线粒体功能障碍。PANoptosis引起的炎症和心肌细胞损失导致心房重构和房颤。HFpEF心肌细胞中NAD+的缺失下调线粒体拓扑异构酶(TOP3A和TOP1MT)和线粒体DNA (mtDNA)应激,促进mtZ-DNA的形成。ZBP1通过其Zα1结构域检测和稳定Z-DNA,并招募受体相互作用蛋白激酶(RIPKs)和Caspase8组装PANoptosome并启动PANoptosis。沉默Zbp1可减轻心房重构,降低心房颤动易感性。此外,补充NAD+通过改善线粒体功能障碍抑制Z-DNA的形成和ZBP1的激活。这些发现确定ZBP1是线粒体功能障碍和PANoptosis之间的分子桥梁,突出了其在hfpef相关AF发病机制中的核心作用。以该轴为靶点可能是治疗HFpEF中房颤的一种有前景的治疗策略。结论通过检测线粒体Z-DNA, ZBP1是连接线粒体功能障碍和PANoptosis的分子桥梁,在hfpef相关AF发病机制中发挥了重要作用。以该轴为靶点可能是治疗HFpEF中房颤的一种有前景的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mitochondrial dysfunction drives ZBP1-mediated PANoptosis to increase the susceptibility of heart failure with preserved ejection fraction-associated atrial fibrillation
Introduction
Heart failure with preserved ejection fraction (HFpEF) is frequently complicated by atrial fibrillation (AF), but underlying molecular mechanisms remain poorly defined. Mitochondrial dysfunction drives ZBP1-mediated PANoptosis is crucial in understanding the progression of HFpEF-associated AF and exploring novel therapeutic avenues.Objectives
This study investigates the Z-DNA binding protein 1 (ZBP1) as a critical mediator linking mitochondrial dysfunction with PANoptosis by sensing mitochondrial Z-DNA (mtZ-DNA) in HFpEF-associated AF.Methods
Variety of in vivo and in vitro experimental approaches were employed, majorly including HFpEF mouse model establishment, Histological staining, RNA sequencing, Western blotting, co-immunoprecipitation, Transmission electron microscopy (TEM) and confocal imaging.Results
In a “Two-hit” HFpEF mouse model, we observed increased AF susceptibility with prolonged modeling. Additionally, bioinformatics analysis and in vivo and in vitro studies highlighted progressive ZBP1-mediated PANoptosis accompanied by mitochondrial dysfunction in HFpEF atria. Inflammation and cardiomyocyte loss caused by PANoptosis contributed to atrial remodeling and AF. Also, NAD+ depletion in HFpEF cardiomyocytes downregulated mitochondrial topoisomerases (TOP3A and TOP1MT) and mitochondrial DNA (mtDNA) stress, promoting mtZ-DNA formation. ZBP1 sensed and stabilized Z-DNA via its Zα1 domain and recruited Receptor-Interacting Protein Kinases (RIPKs) and Caspase8 to assemble the PANoptosome and initiate PANoptosis. Silencing Zbp1 alleviated atrial remodeling and reduced AF vulnerability. Moreover, NAD+ supplementation suppressed Z-DNA formation and ZBP1 activation by improving mitochondrial dysfunction. These findings identify ZBP1 as a molecular bridge between mitochondrial dysfunction and PANoptosis, highlighting its central role in HFpEF-associated AF pathogenesis. Targeting this axis may provide a promising therapeutic strategy combatting AF in HFpEF.Conclusions
These findings identify ZBP1 as a molecular bridge between mitochondrial dysfunction and PANoptosis by sensing mitochondrial Z-DNA, highlighting its central role in HFpEF-associated AF pathogenesis. Targeting this axis may provide a promising therapeutic strategy combatting AF in HFpEF.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


