发现一种新的基于苯并呋喃-7-羧酰胺的PARP1/c-Met双抑制剂,用于解决c-Met扩增介导的PARP1i获得性耐药
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
基于合成致死性原则,PARP1是癌症治疗中一个公认的治疗靶点。然而,PARP1抑制剂的耐药发展显著降低了其抗肿瘤功效,如通过c-Met扩增介导的耐药。在这项研究中,我们报道了一种新的PARP1/c-Met双抑制剂的开发,该抑制剂来源于PARP1抑制剂Mefuparib的苯并呋喃-7-carboxamide部分。与前期研究中获得的化合物16相比,化合物S12具有较低的分子量和较好的溶解度,是一种高效的双抑制剂,对PARP1 (IC50 = 21.8 nM)和c-Met (IC50 = 30.2 nM)均有较强的抑制活性。重要的是,S12在HCT116OR异种移植模型中表现出显著的抗肿瘤疗效,这表明同时靶向PARP1和c-Met是克服c-Met扩增介导的PARP1抑制剂耐药性的有效治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
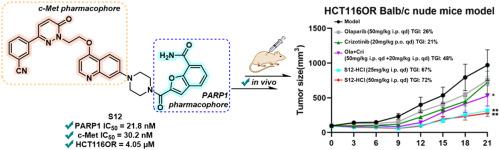
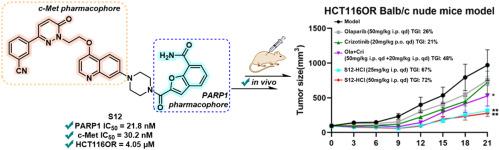
Discovery of a novel benzofuran-7-carboxamide-based PARP1/c-Met dual inhibitor for addressing c-Met amplification-mediated PARP1i acquired-resistance
PARP1 is a well-established therapeutic target in cancer treatment, based on the principle of synthetic lethality. However, the development of drug resistance has significantly compromised the antitumor efficacy of PARP1 inhibitors, exemplified by resistance mediated through c-Met amplification. In this study, we report the development of a novel PARP1/c-Met dual inhibitor derived from the benzofuran-7-carboxamide moiety of the PARP1 inhibitor Mefuparib. Compared with compound 16 obtained in the previous study, compound S12 was identified as a highly potent dual inhibitor with lower molecule weight and better solubility, demonstrating robust inhibitory activity against both PARP1 (IC50 = 21.8 nM) and c-Met (IC50 = 30.2 nM). Importantly, S12 exhibited remarkable anti-tumor efficacy in the HCT116OR xenograft models, suggesting that targeting both PARP1 and c-Met represents an effective therapeutic strategy to overcome PARP1 inhibitor resistance mediated by c-Met amplification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


