自动3D打印儿科速尿片:使用半固体挤压和近红外监测的个性化医疗方法。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究介绍了利用半固态挤压(SSE) 3D打印技术与复合系统解决方案(CSS)技术相结合,开发个性化的儿科速释呋塞米片剂。使用偏最小二乘(PLS)建模的近红外(NIR)光谱,通过傅里叶变换红外光谱(FTIR), x射线衍射(XRD)和扫描白光干涉(SWLI)表面表征来实现剂量个性化和实时质量保证。速尿制剂(1%和2% w/w)使用凝胶为基础的赋形剂制备,并以2至10 mg的剂量印刷。基于nir的PLS模型具有很强的预测精度(R² = 0.91;RMSEC = 3.37%),能够有效、无损地监测混合均匀性。所有制剂均符合欧洲药典对药物含量(85.0-115.0%)和含量均匀性(AV < 15)的要求。溶出度测试证实了快速释放,所有新配制的片剂释放率为85%。6个月后,2%的配方保持了足够的性能(88.5%),而1%的配方表现出中度下降(76.3%)。FTIR和XRD分析显示,药物与赋形剂之间没有明显的相互作用,并且在整个储存过程中,速尿的晶体结构保持完整。SWLI证明了不同配方的表面形貌变化,揭示了赋形剂和表面活性剂水平影响微形貌和潜在的药物释放动力学。SSE 3D打印与光谱和成像工具的集成为个性化儿科药物制造提供了一个强大的、可重复的、以患者为中心的平台。该方法支持向自动化、非无菌配药过渡,具有实时控制、提高剂量精度和提高产品质量的特点,解决了儿科药学服务中长期存在的差距。本文章由计算机程序翻译,如有差异,请以英文原文为准。
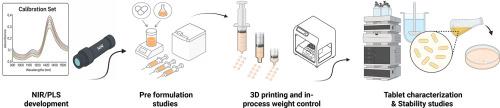
Automated 3D printing of pediatric furosemide tablets: A personalized medicine approach using semi-solid extrusion and NIR monitoring
This study presents the development of personalized, immediate-release furosemide tablets for pediatric use using semi-solid extrusion (SSE) 3D printing integrated with compounding system solution (CSS) technology. Dose personalization and real-time quality assurance were implemented using near-infrared (NIR) spectroscopy with partial least squares (PLS) modeling, supported by Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and surface characterization via scanning white light interferometry (SWLI).
Furosemide formulations (1 % and 2 % w/w) were prepared using a gel-based excipient and printed in doses from 2 to 10 mg. The NIR-based PLS model exhibited strong predictive accuracy (R² = 0.91; RMSEC = 3.37 %), enabling effective, non-destructive blend uniformity monitoring. All formulations met European Pharmacopoeia requirements for drug content (85.0–115.0 %) and content uniformity (AV < 15). Dissolution testing confirmed rapid release profiles, with >85 % release for all freshly prepared tablets. After six months, the 2 % formulation retained adequate performance (88.5 %), while the 1 % formulation showed a moderate decline (76.3 %).
FTIR and XRD analyses revealed no significant drug–excipient interactions, and the crystalline structure of furosemide remained intact throughout storage. SWLI demonstrated surface morphology variations between formulations, revealing that excipient and surfactant levels influenced microtopography and potentially drug release kinetics.
The integration of SSE 3D printing with spectroscopic and imaging tools offers a robust, reproducible, and patient-centric platform for personalized pediatric drug manufacturing. This approach supports the transition toward automated, non-sterile compounding with real-time control, improved dose precision, and enhanced product quality—addressing long-standing gaps in pediatric pharmaceutical care.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


